Draw The Electron Configuration For A Neutral Atom Of Neon
Draw The Electron Configuration For A Neutral Atom Of Neon - Electrons are represented by dots or. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. There are 3 steps to solve this one. Previously we discussed the concept of electron shells, subshells,. Web april 8, 2024 by deep. Using only the periodic table; Describe how electrons are arranged in an atom using electron configurations. When looking at electron configuration, your fill order of electrons is: Describe how electrons are grouped within atoms. Neon has atomic number 10, so a neon atom. This problem has been solved! Draw the electron configuration for a neutral atom of sodium. 100% (9 ratings) share share. Neon has atomic number 10, so a neon atom. How do electron configurations correspond to the periodic table? This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web electron configurationthe arrangements of electrons above the last (closed shell) noble gas. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. There are 3 steps to solve this one. Justify. Draw the electron configuration for a neutral atom of neon. The atomic number of neon is 10. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web intro to electron configurations; Neon electron configuration | image: There are 3 steps to solve this one. Determine the electron configuration of ions. Web april 8, 2024 by deep. In almost all cases, chemical bonds are formed by interactions of valence electrons in. Web chemistry electron configuration electron configuration. 100% (9 ratings) share share. Describe how electrons are arranged in an atom using electron configurations. The atomic number of neon is 10. Using only the periodic table; Web electron configurationthe arrangements of electrons above the last (closed shell) noble gas. Web intro to electron configurations; Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Draw the electron configuration for a neutral atom of neon. Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is. Neon has atomic number 10, so a neon atom. Then, add or remove electrons depending on the ion's charge.
Electron Configuration Chart With Orbitals
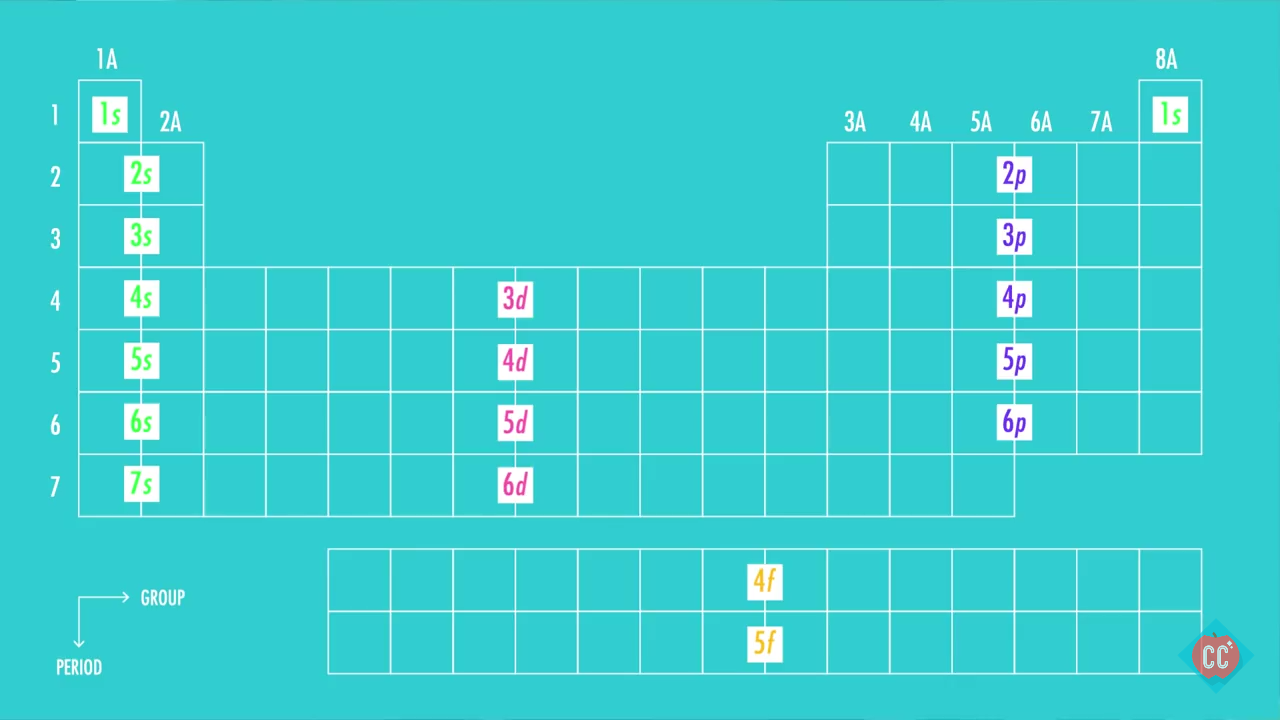
What is the groundstate electron configuration of a neutral atom of
![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)
[5 Steps] Electron Configuration for or of Neon in Just 5 Steps
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Web To Find The Electron Configuration For An Ion, First Identify The Configuration For The Neutral Atom.
Draw The Electron Configuration For A Neutral Atom Of Sodium.
The Neon Electron Configuration, Represented As [ He].
Related Post: