Draw The Electron Configuration For A Neutral Atom Of Magnesium
Draw The Electron Configuration For A Neutral Atom Of Magnesium - Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Click within an orbital to add electrons. The electron configuration for the magnesium. It's best to have two p 63 s two. You'll get a detailed solution from a subject matter expert. Web the ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Want to join the conversation? Draw the electron configuration for a neutral atom of magnesium; Subshells are described by writing the principal quantum number n followed by the symbol for the angular momentum quantum number l (s, p, d, or f). The shorthand electron configuration for magnesium is [ne] 3s 2. Energy 1 l x х ? The aufbau diagram can help you determine the correct order in which the electrons will fill the orbitals in an atom. In shorthand notation, it can be written as [ne] 3s 2 ^2 2. Web therefore the magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Web electron affinity the. Web therefore the magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Draw the electron configuration for a neutral atom of magnesium; Web electron affinity the energy released when an electron is added to the neutral atom and a. Find all video solutions for your textbook. This video solution was recommended by our tutors as helpful. Web the electron configuration of a neutral magnesium atom is: Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. Energy x 6 2 place orbital. This is the electron configuration of helium; Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg use the buttons. You'll get a detailed solution from a subject matter expert. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. The question wants us to write down the electronic configuration for magnesium, which is going to be one us to two. Atomic number, atomic weight and charge of magnesium ion. Web the electron configuration of a neutral magnesium atom is: Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Web construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg. Web the electron configuration for a neutral atom of mg is 1s 2 ^2 2 2s 2 ^2 2 2p 6 ^6 6 3s 2 ^2 2. The configuration notation provides an easy way for scientists to write and communicate how. This problem has been solved! Not all targets will be filled. Want to join the conversation? In shorthand notation, it can be written as [ne] 3s 2 ^2 2. 1s2 2s2 2p6 3s2 or in shorthand [ne] 3s2. This is the electron configuration of helium; Web therefore the magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.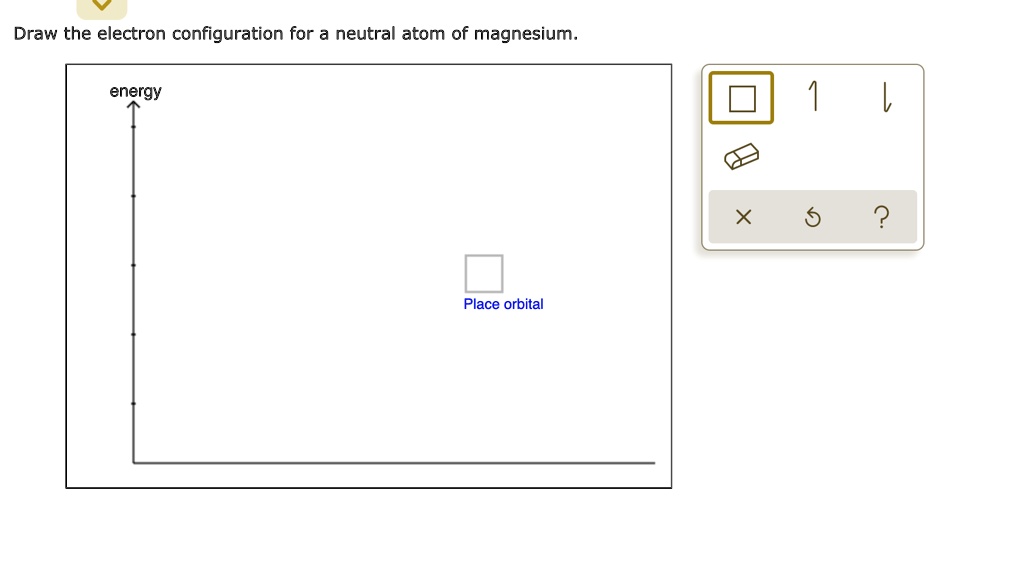
SOLVED Draw the electron configuration for a neutral atom of magnesium
:max_bytes(150000):strip_icc()/magnesiumatom-58b6026b5f9b5860464c7467.jpg)
Atoms Diagrams Electron Configurations of Elements
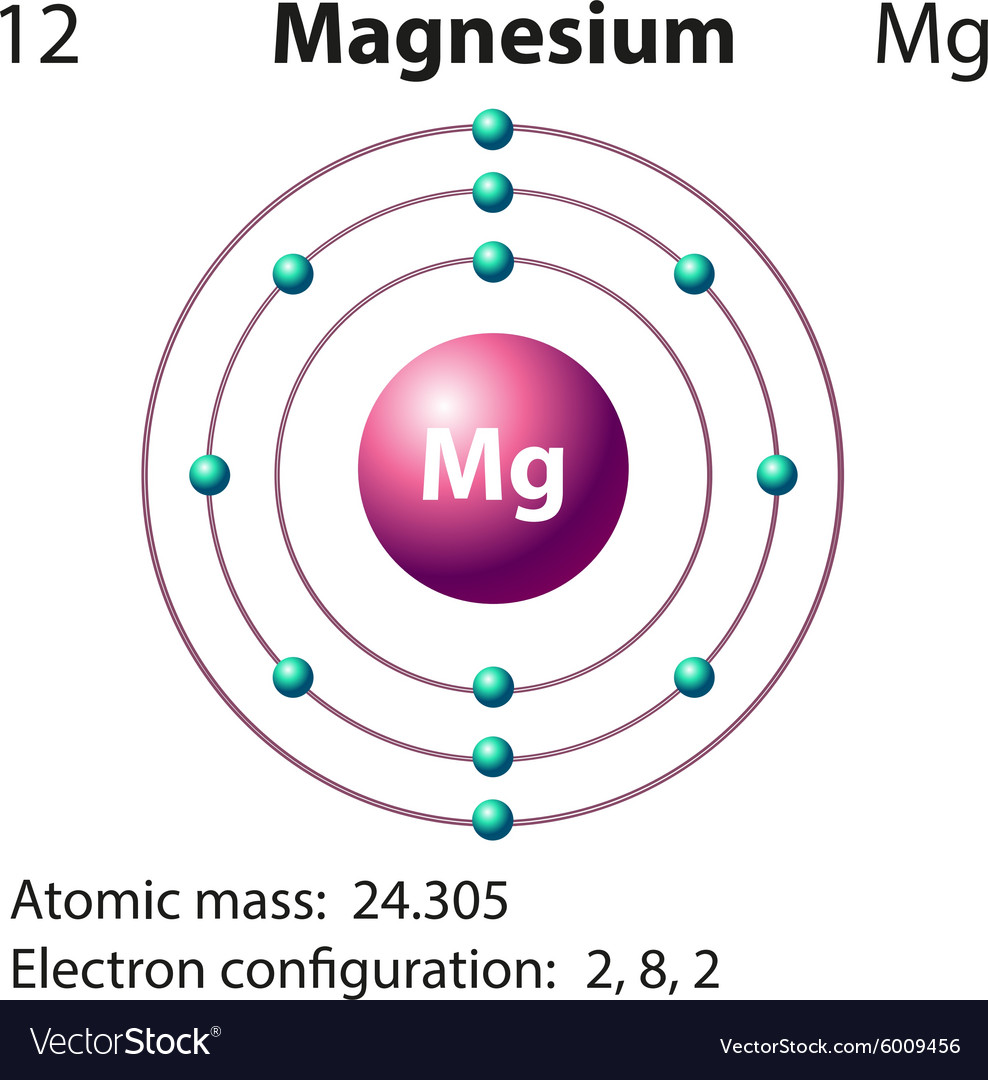
Diagram representation of the element magnesium Vector Image
Subshells Are Described By Writing The Principal Quantum Number N Followed By The Symbol For The Angular Momentum Quantum Number L (S, P, D, Or F).
Draw The Electron Configuration For A Neutral Atom Of Magnesium;
Here Are A Few Highlights That You Learned In The Last Section About Bohr's Model:
It Denotes A Full S Orbital.
Related Post: