Draw The Electron Configuration For A Neutral Atom Of Helium
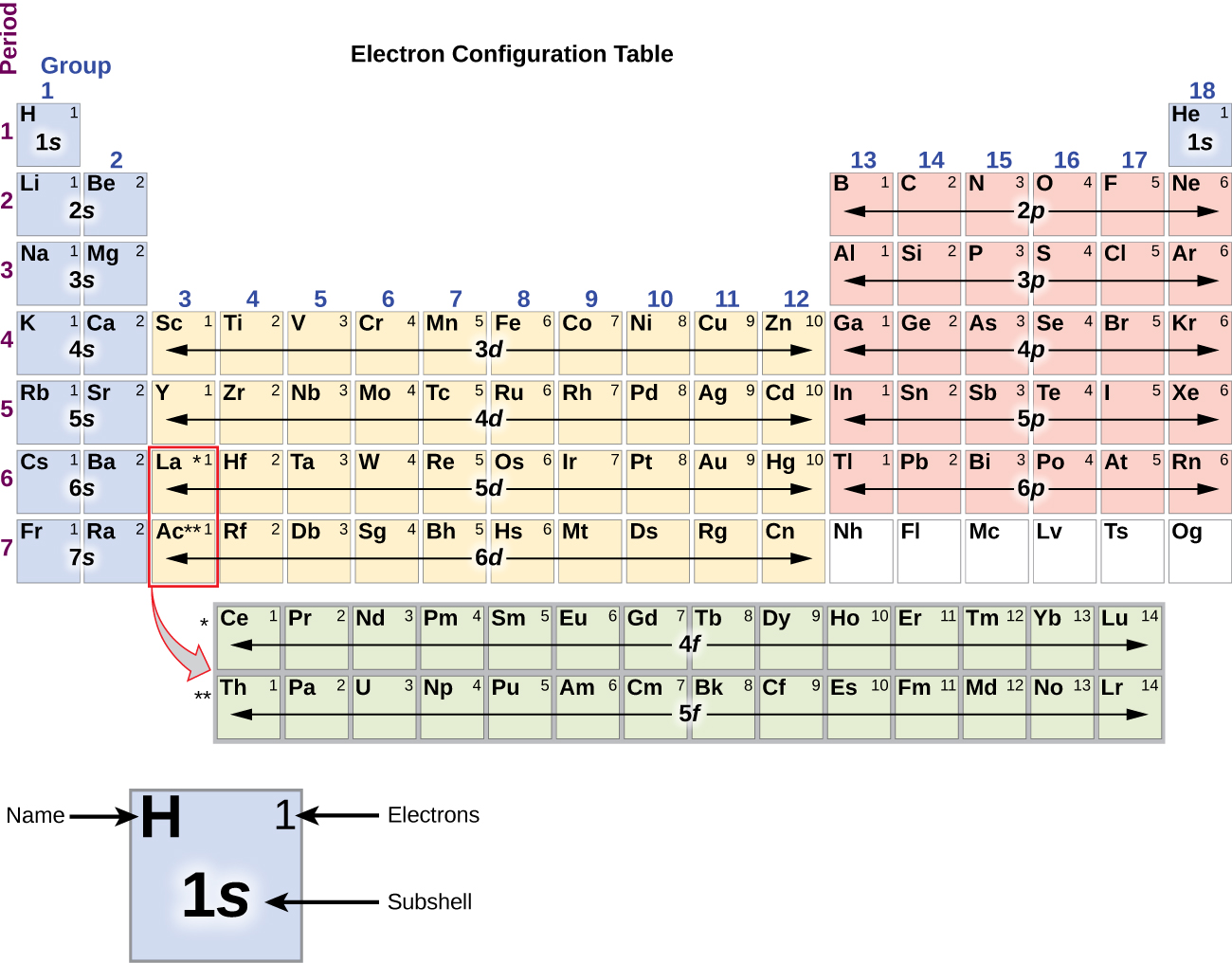
Draw The Electron Configuration For A Neutral Atom Of Helium - We place one electron in the orbital that is lowest in energy, the 1 s orbital. Web helium, #he#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus. Energy 1 l х 5 ? This problem has been solved! Draw the electron configuration for a neutral atom of aluminum. Web chemistry questions and answers. Helium atoms have 2 electrons. This problem has been solved! This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Draw a lewis electron dot diagram for an atom or a monatomic ion. Energy 1 l х 5 ? Draw the electron configuration for a neutral atom of helium. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) helium atom (bohr model) electron configuration through orbitals follows different principles. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Hydrogen. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. From the pauli exclusion principle , we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing. Draw the electron configuration for a neutral atom of helium. So,. Web the electron configuration of helium is 1s2, which means there are two electrons in the 1s orbital. Draw a lewis electron dot diagram for an atom or a monatomic ion. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To facilitate our understanding of how valence electrons interact, a simple way of. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration can be done in two ways. It denotes a full s orbital. Make certain that you can define, and use in context, the key terms below. Using only the periodic table; Energy 1 i x $ ? This is the electron configuration of helium; This problem has been solved! Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. This problem has been solved! The helium atom contains two protons and two electrons. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. To draw the electron configuration diagram for helium, we can represent the first energy level as a circle and place two dots inside it. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons.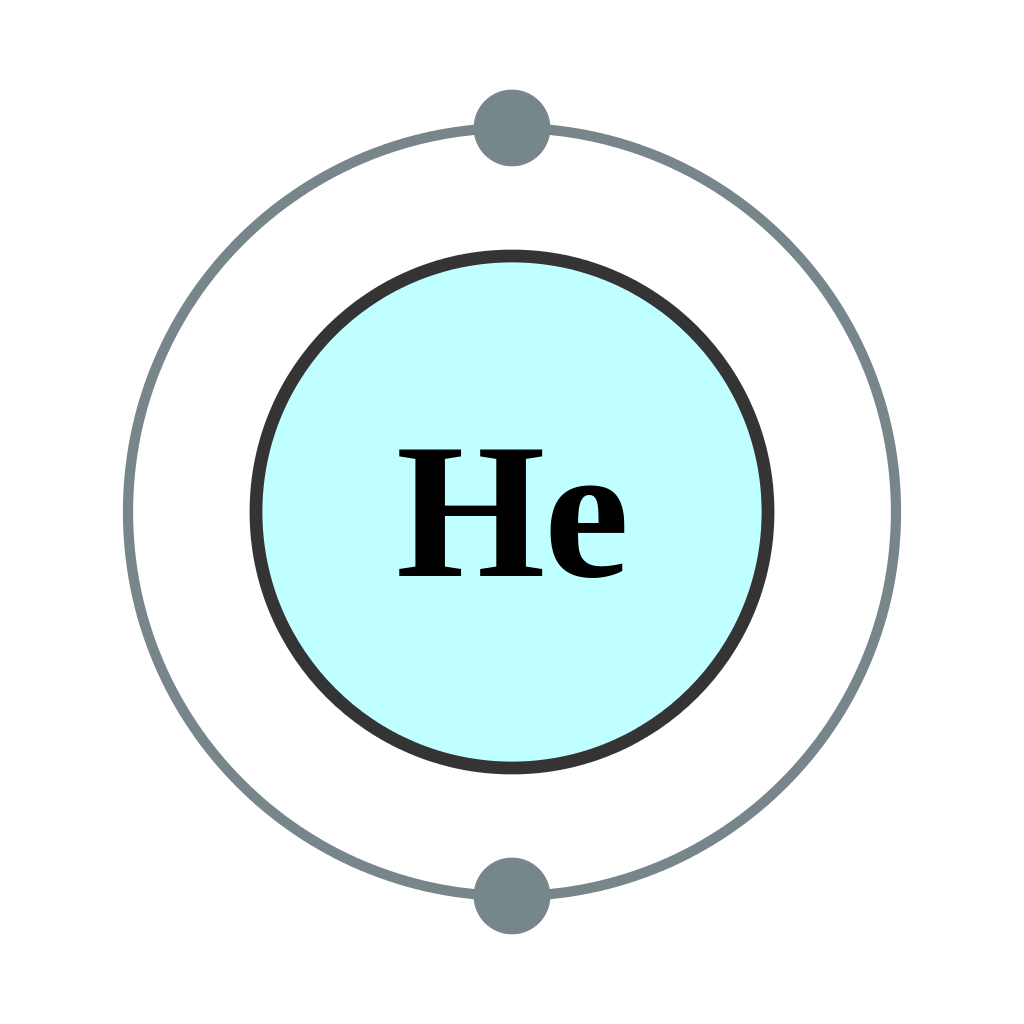
How To Find the Helium Electron Configuration (He)
Helium electric configuration saildop

Helium, atomic structure Stock Image C018/3683 Science Photo Library
Web The Upper Right Side Shows The Number Of Electrons In A Neutral Atom.
Draw The Electron Configuration For A Neutral Atom Of Aluminum.
Draw The Electron Configuration For A Neutral Atom Of Aluminum.
Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O) Fluorine (F) Neon (Ne) Sodium (Na) Magnesium (Mg) Aluminum (Al) Silicon (Si.
Related Post: