Draw The Electron Configuration For A Neutral Atom Of Fluorine
Draw The Electron Configuration For A Neutral Atom Of Fluorine - Fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs. Draw the electron configuration for a neutral atom of fluorine. Web for example, the electron configuration of fluorine (f) is 1s^2 2s^2 2p^5, indicating that it has two electrons in the 1s orbital, two electrons in the 2s orbital, and five electrons in. Fluorine belongs to group 17 which is known as halogens. Web one electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. 1s22s22p5 draw the lewis dot symbol for a neutral fluroine atom. Web what is the electron configuration of: Web similarly, fluorine has the electron configuration 1 s2 2 s2 2 p5: Web the neutral atom chlorine (z=17), for instance has 17 electrons. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web one electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Web fluorine is the most electronegative element because it has 5 electrons in its 2p shell. Then, add or remove electrons depending on the ion's charge. Web to find the. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the electron configuration for a neutral atom of fluorine. Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Write the full electron configuration for a neutral fluorine atom. Web for example, the electron configuration of fluorine (f). Draw the electron configuration for a neutral atom of fluorine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1s22s22p5 draw the lewis dot symbol for a neutral fluroine atom. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the. 1s22s22p5 draw the lewis dot symbol for a neutral fluroine atom. This problem has been solved! Electronic configuration of group 17:. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the. Draw the electron configuration for a neutral atom of fluorine. Web the general notation of electronic configuration is s a p b d c where s, p, and d represents the subshell and a, b and c represents the number of electrons present in the subshell. Fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs. Web the electron configuration of fluorine is: Draw the electron configuration for a neutral atom of fluorine. Web introduction to chemistry (chm 200) 2: Write the full electron configuration for a neutral fluorine atom. You'll get a detailed solution. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Then, add or remove electrons depending on the ion's charge. In this article, we will study how electrons are arranged in different shells and subshells in the fluorine atom. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: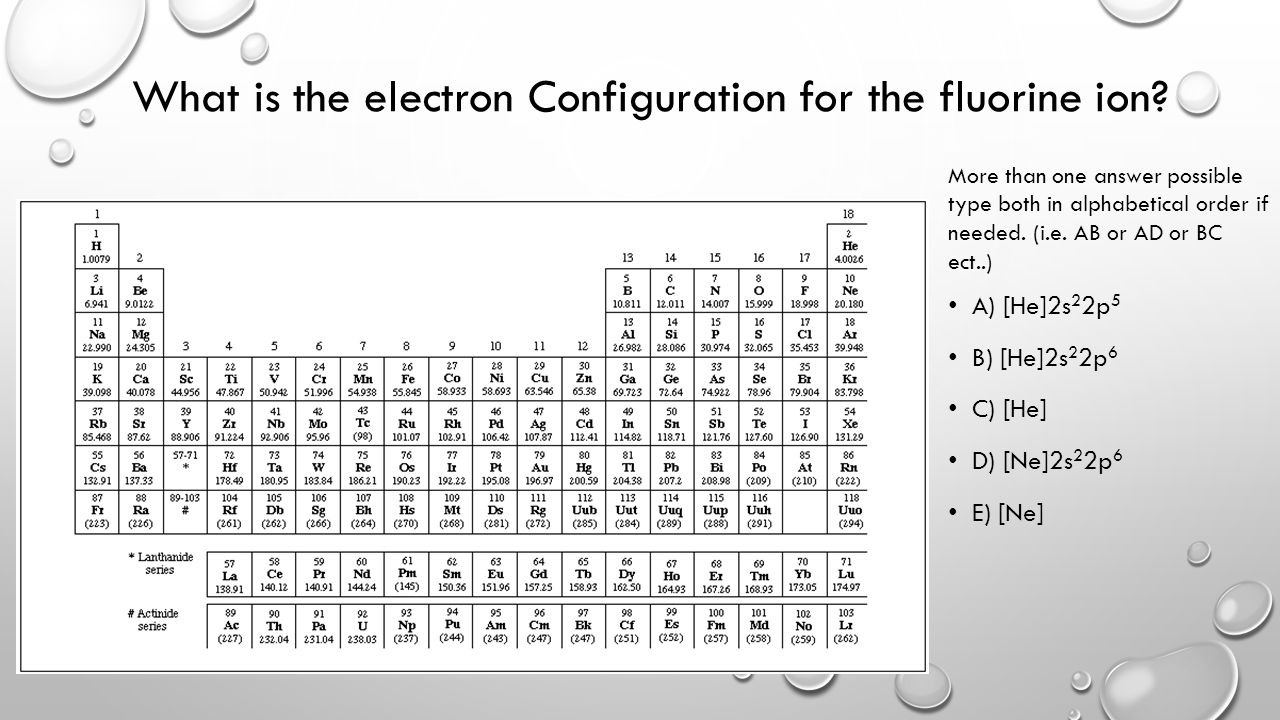
How To Find Electron Configuration For Fluorine Dynamic Periodic

fluorine orbital diagram Herbalned

Diagram representation element fluorine Royalty Free Vector
Web Fluorine (Atomic Number 9) Has Only One 2 P Orbital Containing An Unpaired Electron.
Using Only The Periodic Table;
Fluorine Belongs To Group 17 Which Is Known As Halogens.
Web Fluorine Is The Most Electronegative Element Because It Has 5 Electrons In Its 2P Shell.
Related Post: