Draw The Electron Configuration For A Neutral Atom Of Carbon
Draw The Electron Configuration For A Neutral Atom Of Carbon - Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web this raises an interesting question: No electrons remain h 3 cch 3: Web the electron configuration of carbon is [ he] 2s 2 2p 2 , if the electron arrangement is through orbitals. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. How does the sulfur atom in sf 4 hold 10 electrons in its valence shell? In almost all cases, chemical bonds are formed by interactions of valence electrons in. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in. For example, the electron configuration of lithium, 1s²2s¹, tells us that. Energy 0 1 1 x i ? Draw a lewis electron dot diagram for an atom or a monatomic ion. In writing the electron configuration for carbon the first two electrons. For example, the electron configuration of lithium, 1s²2s¹, tells us that. Experimentally, it is found that the. In writing the electron configuration for carbon the first two electrons. Web the electron configuration of carbon is [ he] 2s 2 2p 2 , if the electron arrangement is through orbitals. Web where needed, place remaining electrons on the central atom: In almost all cases, chemical bonds are formed by interactions of valence electrons in. Experimentally, it is found. For example, the electron configuration of lithium, 1s²2s¹, tells us that. Nitrogen (atomic number 7) fills the 1 s and 2 s subshells and has one electron in each of the three 2 p orbitals, in. Electron configurations describe where electrons are located around the nucleus of an atom. Web this raises an interesting question: Web the electron configuration and. Four electrons placed on carbon nh 3: Electron configurations describe where electrons are located around the nucleus of an atom. Electrons are represented by dots or. Web the electron configuration of carbon is [ he] 2s 2 2p 2 , if the electron arrangement is through orbitals. Web how to write the electron configuration for carbon. Experimentally, it is found that the. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Carbon is the sixth element with a total of 6 electrons. Web by hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Web by hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. How does the sulfur atom in sf 4 hold 10 electrons in its valence shell? This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Energy 0 1 1 x i ? Previously we discussed the concept of electron shells,. Web the electron configuration and orbital diagram for carbon are: For example, the electron configuration of lithium, 1s²2s¹, tells us that.
Draw The Electron Configuration For A Neutral Atom, HD Png Download
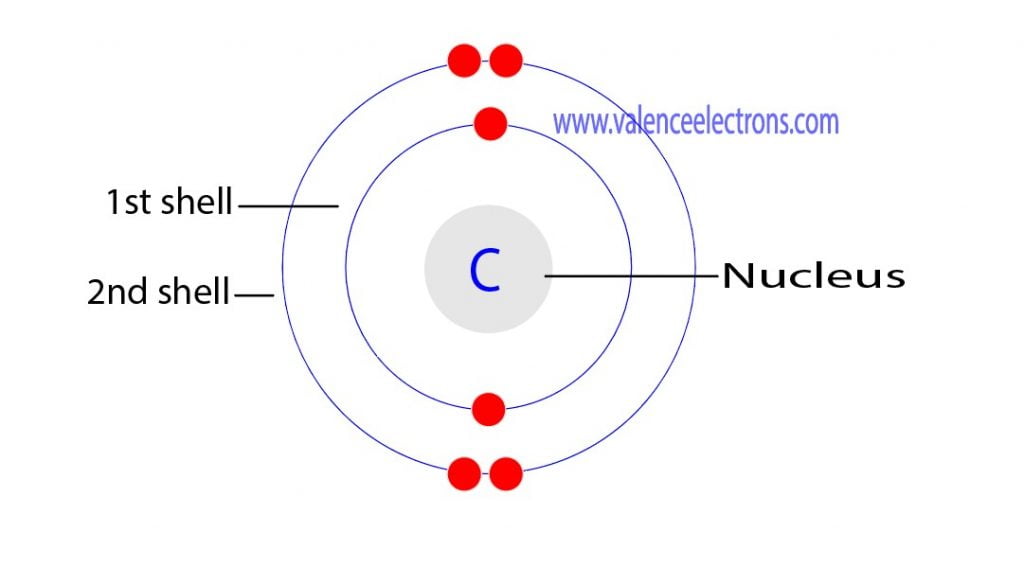
Electron Configuration for Carbon (C, C4−) Full Guide

Carbon electron configuration Stock Image C029/5022 Science Photo
When Writing An Electron Configuration, First Write The Energy Level (The Period), Then The Subshell To Be Filled And The Superscript, Which Is The.
No Electrons Remain H 3 Cch 3:
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Web The Electron Configuration And Orbital Diagram For Carbon Are:
Related Post: