Draw Lewis Structure For So2
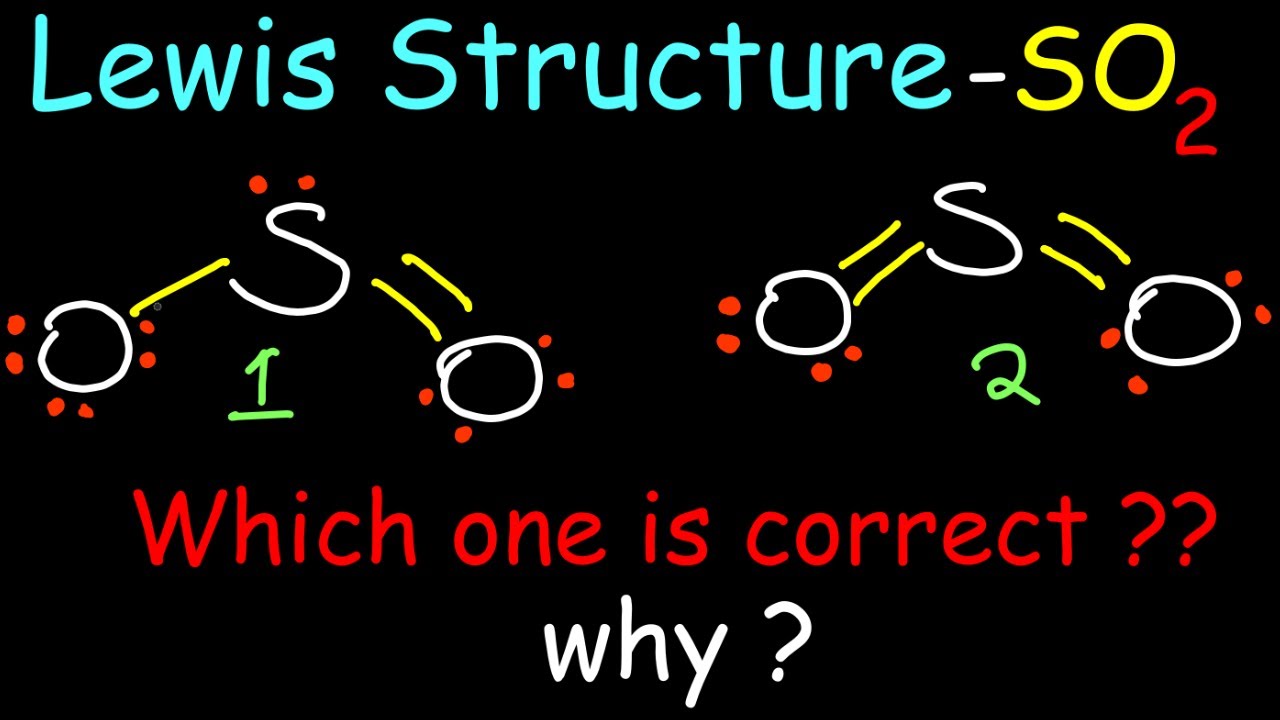
Draw Lewis Structure For So2 - Drawing the lewis structure of so2 is critical for understanding its molecular bonding and chemical characteristics. Calculate the total number of valence electrons. 146k views 3 years ago. To draw the lewis structure of the so a 2 ( sulfur dioxide) molecule, follow these steps: That will normally be the least electronegative atom ( s ). Web how do you draw the lewis structure for sulfur dioxide (so2)? This problem has been solved! This chemistry video tutorial explains how to draw the lewis structure of so2 also known as sulfur dioxide. S and o atoms have sp2 hybridization. The lewis structure predicts the molecular shape, polarity, and reactivity. There are so many facts about the internal structure obtained from drawing of lewis structure. Here, a = central atom, x = surrounding atoms and e = the lone pairs. May 25, 2022 by upasana nayak. There are two oxygen atoms bonded to the central sulfur atom. The valence electrons are the electrons in the. How many valence electrons does sulfur dioxide (so2) have? It discusses the molecular geometry, bond angle,. Note that so2 is a bit. In so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. Start by counting the valence electrons of each atom in the molecule. Draw the best lewis structure for so2 by filling in the bonds, lone pairs, and formal charges. How many valence electrons does sulfur dioxide (so2) have? This problem has been solved! 534k views 10 years ago. Web to determine the molecular geometry of sulfur dioxide, we must observe its lewis structure. Web how do you draw the lewis structure for sulfur dioxide (so2)? Web draw a lewis structure for so2 in which all atoms have a formal charge of zero. That will normally be the least electronegative atom ( s ). 534k views 10 years ago. Web home » basic chemistry. What is the molecular geometry of so2 based on its lewis structure? Web the lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur (s). There are so many facts about the internal structure obtained from drawing of lewis structure. It discusses the molecular geometry, bond angle,. Determine the total valence electrons. Sulfur dioxide lewis structure is drawn step by step using vespr rules. View the full answer answer. Here, the given molecule is so2 (sulfur dioxide). The lewis structure predicts the molecular shape, polarity, and reactivity. So i would assume that the one with two double bonds is the correct structure. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Lewis Structure of Sulphur Dioxide SO2 YouTube

How to draw SO2 Lewis Structure? Science Education and Tutorials
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Note That So2 Is A Bit.
Web The Dot Structure For Sulfur Dioxide Has Sulfur With A Double Bond To An Oxygen On The Left, And Two Lone Pairs Of Electrons On That Oxygen, And The Sulfur With A Double Bond To An Oxygen On The Right, And Two Lone Pairs Of Electrons On That Oxygen.
Web To Determine The Molecular Geometry Of Sulfur Dioxide, We Must Observe Its Lewis Structure.
Related Post: