Draw Lewis Structure For Cs2
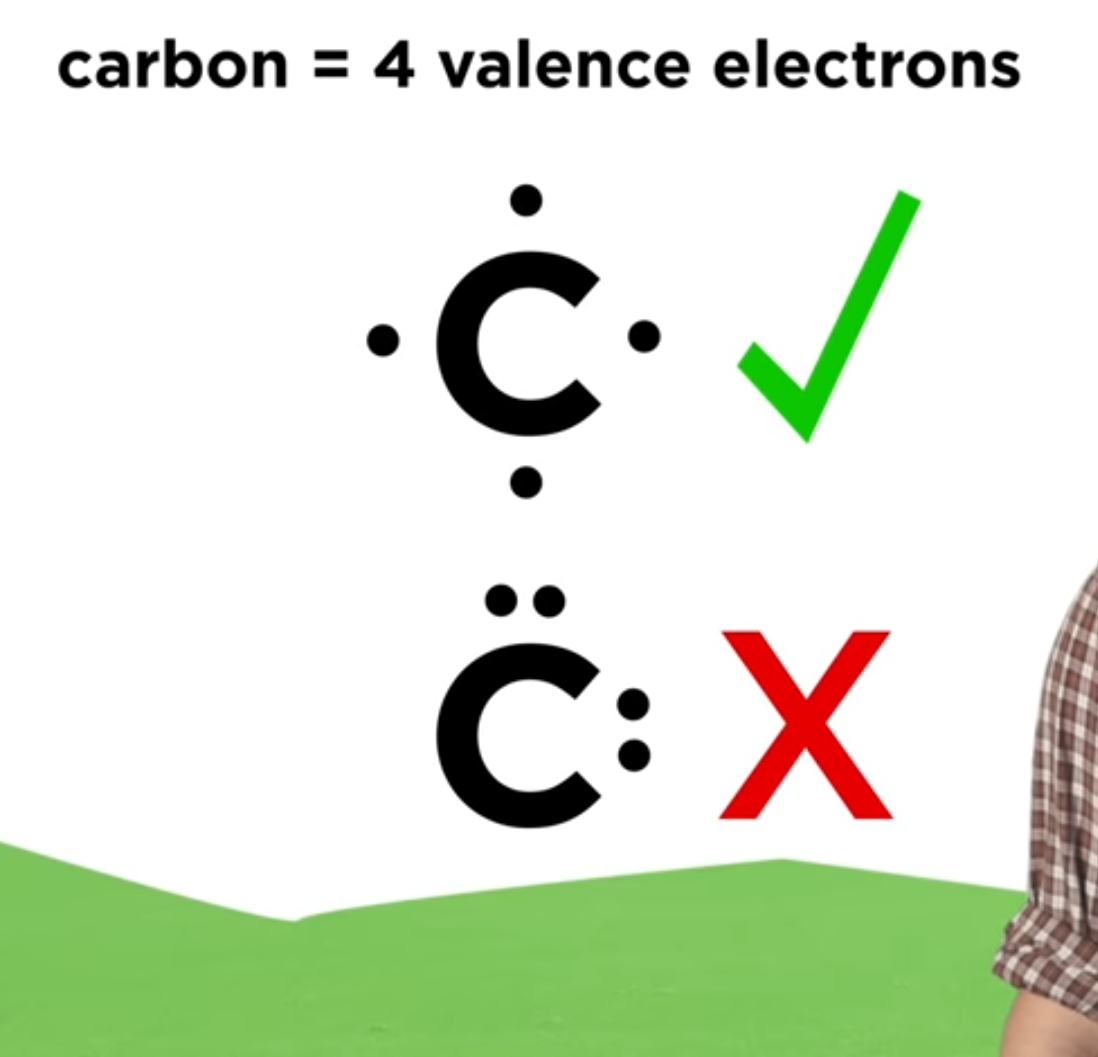
Draw Lewis Structure For Cs2 - For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Send feedback | visit wolfram|alpha. Distribution of remaining valence electrons: After determining how many valence electrons there are in cs2, place them around the central atom to complete the octets. There are 16 valence electrons for the cs2 lewis structure. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. #1 first draw a rough sketch. Lewis structure is the structural representation of the number of valence electrons that participate in the bond formation and nonbonding electron pairs. Draw a lewis structure for. Here, the given molecule is cs2 (carbon disulfide). Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Connect the atoms with dots: Lewis structure is the structural representation of the number of valence electrons that participate in the bond formation and nonbonding electron pairs. There are 16 valence electrons for the cs2 lewis structure. #5 repeat step 4 if needed, until all. Web lewis structure for cs2 (carbon disulfide) commonly tested lewis structures. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Could u guys help me with q #2 ? Lewis structure is the structural representation of the number of valence electrons that participate in the bond formation and nonbonding electron pairs. Web steps of. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. #2 mark lone pairs on the atoms. To draw a lewis structure, dots and lines are used in this structu. Calculate the total number of valence electrons. Here, the given molecule is cs2 (carbon disulfide). We draw lewis structures to predict: Web drawing the lewis structure for cs 2. After determining how many valence electrons there are in cs2, place them around the central atom to complete the octets. Find more chemistry widgets in wolfram|alpha. The valence electrons are the electrons in the. #3 calculate and mark formal charges on the atoms, if required. Write the correct skeletal structure for the molecule. Carbon (c) possesses 4 valence electrons, while sulfur (s) has 6 valence electrons. Determine the central metal atom: For the cs2 lewis structure, calculate the total number of valence electrons for the cs2 molecule. Send feedback | visit wolfram|alpha. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. There are 16 valence electrons for the cs2 lewis structure. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. To draw a lewis structure, dots and lines are used in this structu. Web 6 steps of cs2 lewis structure: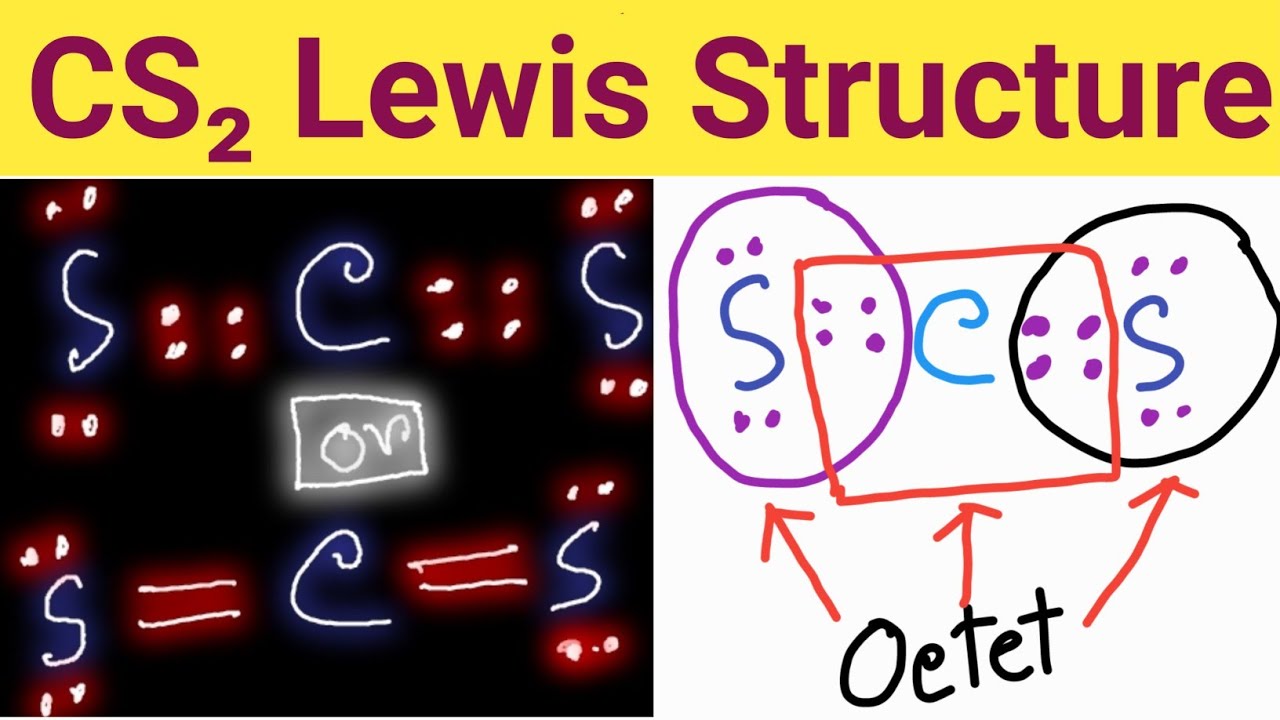
CS2 Lewis Structure Lewis Dot Structure for CS2 Carbon Disulfide
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

So far, we’ve used 16 of the CS2 Lewis structure’s total 16 outermost
Draw A Lewis Structure For.
Web In Short, These Are The Steps You Need To Follow For Drawing A Lewis Structure:
Cs2 Has Two S Atoms, Hence, The Valence Electrons In Sulfur Here Are 6*2=12.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post: