Draw Lewis Structure For Ch2O
Draw Lewis Structure For Ch2O - A lewis structure is a way to show how atoms share electrons when they form a molecule. Web the lewis diagram of a molecule can be constructed using the following stepwise procedure: In order to find the total valence electrons in ch2o molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. It is twelve as two are coming from the two hydrogen atoms, four from the. Web added jun 9, 2014 by webtester in chemistry. 91k views 12 years ago every video. 1.) count up the total number of valence electrons available. Web draw lewis structures depicting the bonding in simple molecules. (2) draw single bonds between bonded atoms. The valence electrons are the electrons in the. 91k views 12 years ago every video. Send feedback | visit wolfram|alpha. Find more chemistry widgets in wolfram|alpha. How to draw lewis structure for ch 2 o; #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure. In all cases, these bonds involve the sharing or transfer of. Web the lewis structure of ch2o is drawn as: Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web © 2024 google llc. It is a chemical formula for methanol or what is also commonly referred to a. I quickly take you through how to draw the lewis structure of ch2o (methanal or formaldehyde). Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Let’s draw and understand this lewis dot. So, the total number of valence electrons in ch2o is: For very simple molecules and molecular ions, we. Looking at the periodic table, carbon has 4. Web to draw lewis structures. Draw lewis structures for molecules. How to draw lewis structure for ch 2 o; = 12 valence electrons of ch2o. The oxygen atom has 2 lone pairs. The carbon atom (c) is at the center and it is surrounded by two hydrogen (h) and one oxygen atom (o). Lewis structure is a pictorial representation of the atoms in the molecules, their bonds, and lone pairs of electrons. Web the lewis structure of ch2o is drawn as: Web how to draw the lewis structure for ch2o. Web learn the steps to draw the lewis structure of ch2o (formaldehyde) in just 1 minute.📌you can draw any lewis structures by following the simple steps mention. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. The number of valence electrons for carbon is 4, oxygen is 6, and hydrogen is 1. Web the structure is made up of electrons drawn as dots, mostly in pairs, around the atom symbol. 4 (c) + 2 (h) + 6 (o) = 12. What is the strongest intermolecular force this molecule wo exhibit?
Draw the best Lewis structure for CH2O Based on the Lewis structure for

How to Draw the Lewis Dot Structure for CH2O (Formaldehyde) YouTube
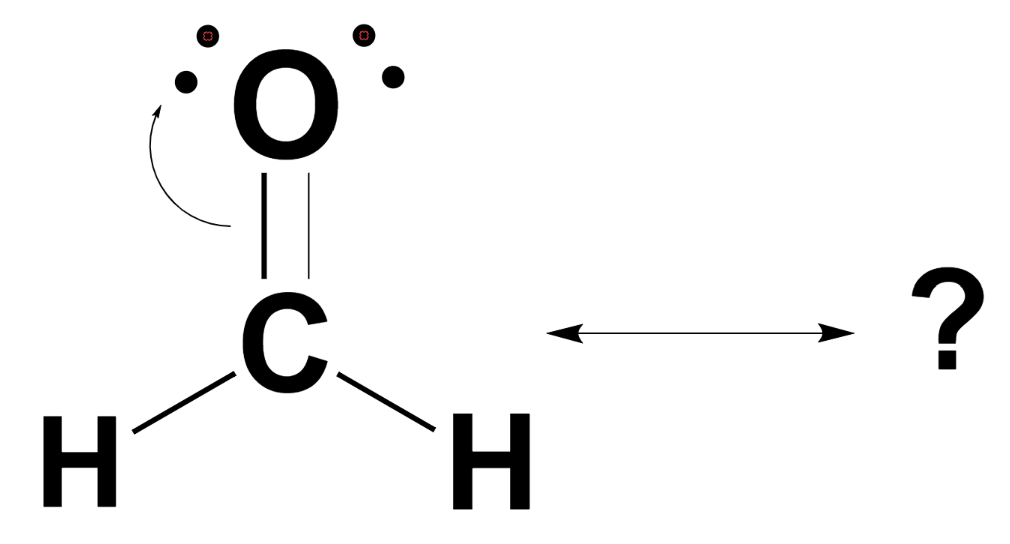
Lewis Structure Of Ch2o
Search For The Total Already Available Valence Electrons In A Single Formaldehyde Ch2O Molecule :
Carbon (C) Is The Least Electronegative Atom In The Ch2O Lewis Structure And Therefore Should Be Placed At.
Let’s Draw And Understand This Lewis Dot.
Drawing The Ch2O Lewis Structure Involves Several Steps:
Related Post: