Draw H2O Molecule
Draw H2O Molecule - 162k views 12 years ago every video. Identify and count the pendant atoms' valence orbitals. The h2o lewis structure shows the two hydrogen atoms bonded to the oxygen atom, and the two unshared electron pairs on the oxygen atom. For h2o, this would be 2 + 6 = 8, since hydrogen has 1 valence electron and oxygen has 6. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web here are the steps to follow while drawing the lewis structure for h 2 o: How to draw lewis structure for h 2 o. Look for how many electrons are needed: Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web how to draw h2o lewis structure. The h2o lewis structure shows the two hydrogen atoms bonded to the oxygen atom, and the two unshared electron pairs on the oxygen atom. Web commonly tested lewis structures. 162k views 12 years ago every video. You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. Oxygen is. We draw lewis structures to predict: You must arrange 8 electrons in pairs so that o has 8 and each h has two electrons in its valence shell. Polarity of h 2 o; Determine the total number of valence electrons for all the atoms in the molecule. Find the total valence electrons in h2o molecule. Find the total valence electrons in h2o molecule. Identify and count the pendant atoms' valence orbitals. I quickly take you through how to draw the lewis structure of water, h2o. Web here are the steps to follow while drawing the lewis structure for h 2 o: In order to find the total valence electrons in h2o molecule, first of all. Look for how many electrons are needed: (valence electrons are the number of electrons present in the outermost shell of an atom). The lewis structure of h2o, or water, can be drawn by following these steps: For h2o, there are 2 valence electrons for each hydrogen atom and 6 valence electrons for the oxygen atom, giving a total of 8 valence electrons. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Calculate the total number of valence electrons. Web lewis structure of water molecule contains two single bonds around oxygen atom. Web steps of drawing h2o lewis structure. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web here are the steps to follow while drawing the lewis structure for h 2 o: (valence electrons are the electrons that are present in the outermost. Web the key to understanding water’s chemical behavior is its molecular structure. In order to draw the lewis structure of h2o, first of all you have to find the total number of valence electrons present in the h2o molecule. To get the total number of valence electrons for this molecule, we will add up hydrogen and oxygen atoms’ valence electrons. Construct salcs and the molecular orbital diagram for h 2 o. Find the point group of the molecule and assign cartesian coordinates so that z is the principal axis.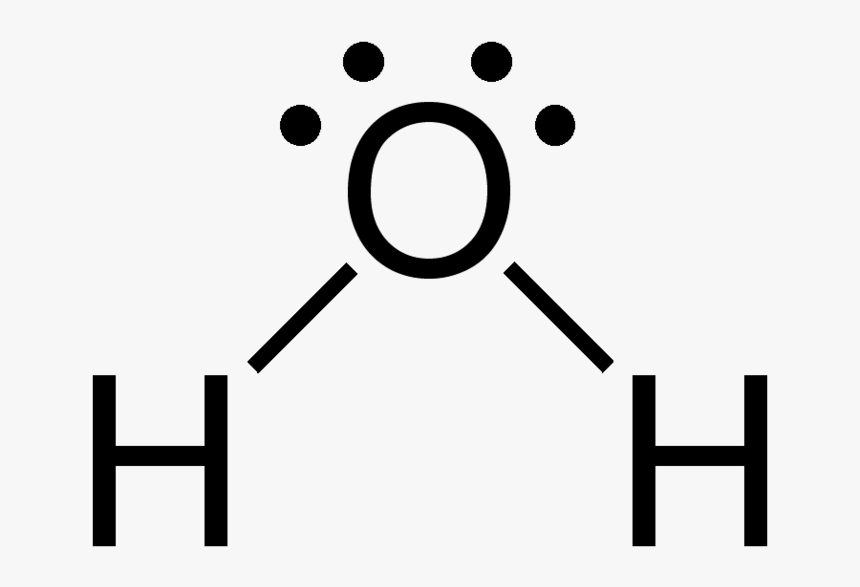
H2o Atomic Structure
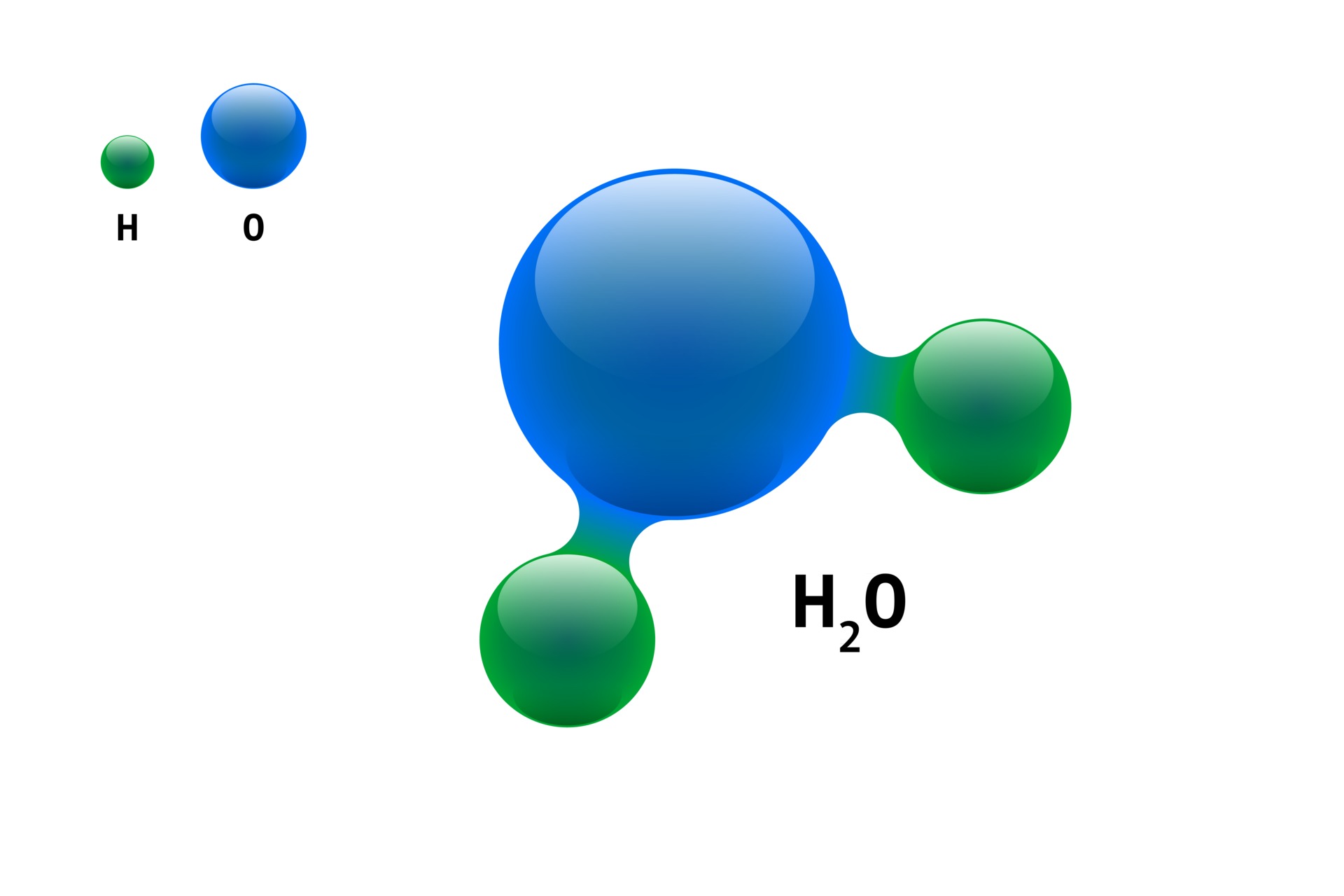
H2O Molecule Model

H2O Lewis Structure, Molecular Geometry, and Hybridization
Find The Total Valence Electrons In H2O Molecule.
A Water Molecule Consists Of Two Hydrogen Atoms Bonded To An Oxygen Atom, And Its Overall Structure Is Bent.
162K Views 12 Years Ago Every Video.
Web Structure Of Water And Hydrogen Bonding.
Related Post: