Draw Dipole Moment
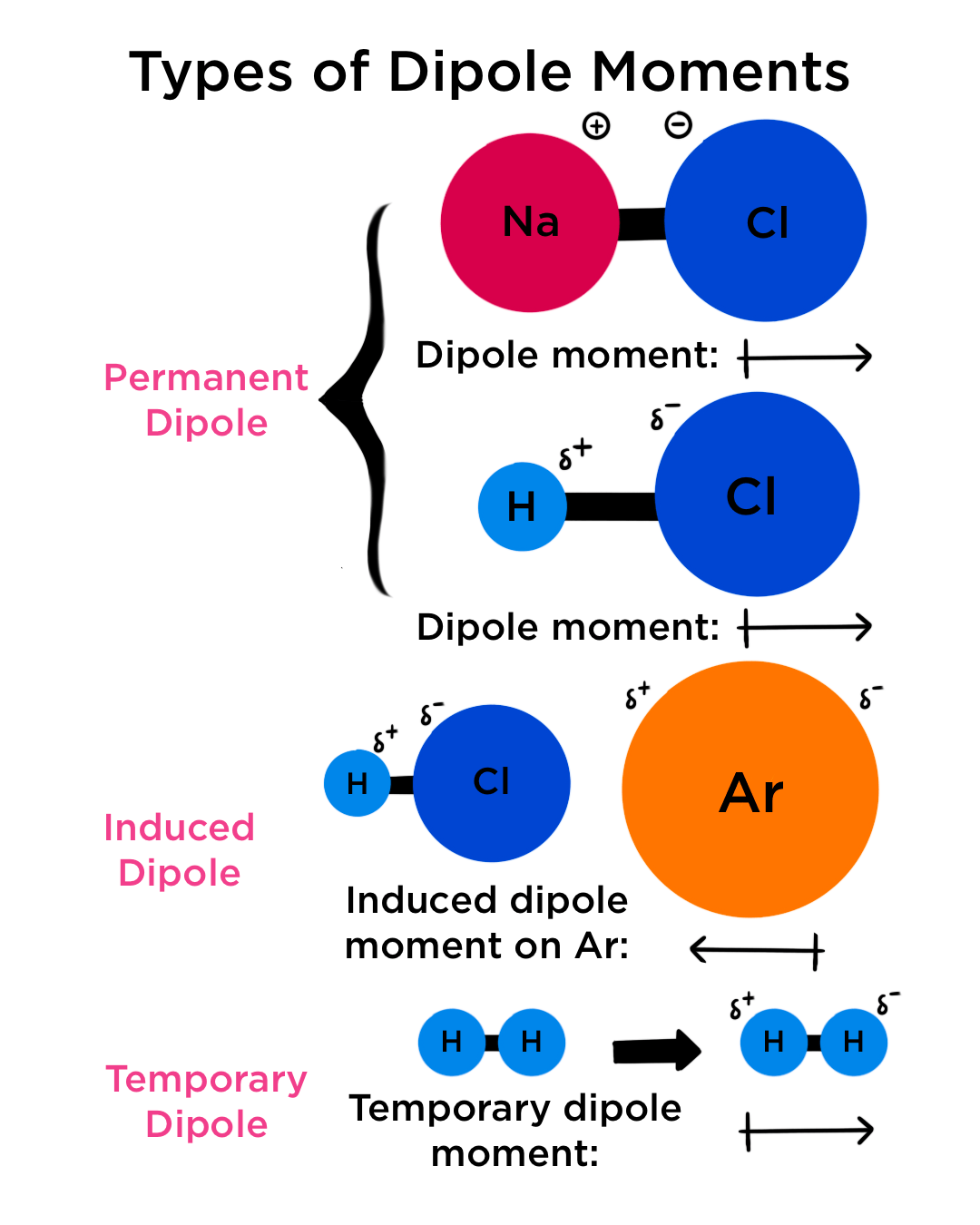
Draw Dipole Moment - The size of a dipole is measured by its. (where is the bond dipole moment, is the magnitude of the separated charge, and is the distance. When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive. The cross base arrow demonstrates the net dipole. Web the dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their dipole moments. The difference in electronegativity can be used to. The separation of charges in any system leads to a dipole moment. Web a moment is a measure of a turning force about an axis. It is denoted by the greek letter ‘µ’. Web a dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. The size of a dipole is measured by its. Web a dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. Both ionic and covalently bonded compounds develop dipole. 9, part 1) jared christensen. Web the dipole moment is a vector quantity, having a magnitude and a. Both ionic and covalently bonded compounds develop dipole. Web the dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their dipole moments. 9, part 1) jared christensen. Dipole moments are often represented as vectors. It explains how to indicate the polarity of a. Looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: The separation of charges in any system leads to a dipole moment. The size of a dipole is measured by its. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, a dipole is established. In general,. It explains how to indicate the polarity of a. When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive. Both ionic and covalently bonded compounds develop dipole. Looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: Web author jayashree raman. The separation of charges in any system leads to a dipole moment. Web the dipole moment is a vector quantity, having a magnitude and a direction. The cross base arrow demonstrates the net dipole. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, a dipole is established. The size of a dipole is measured by its. It is denoted by the greek letter ‘µ’. The difference in electronegativity can be used to. In general, the magnitude of the dipole. Learn how to determine whether a molecule. Learn about carbon dioxide's dipole. Web a moment is a measure of a turning force about an axis.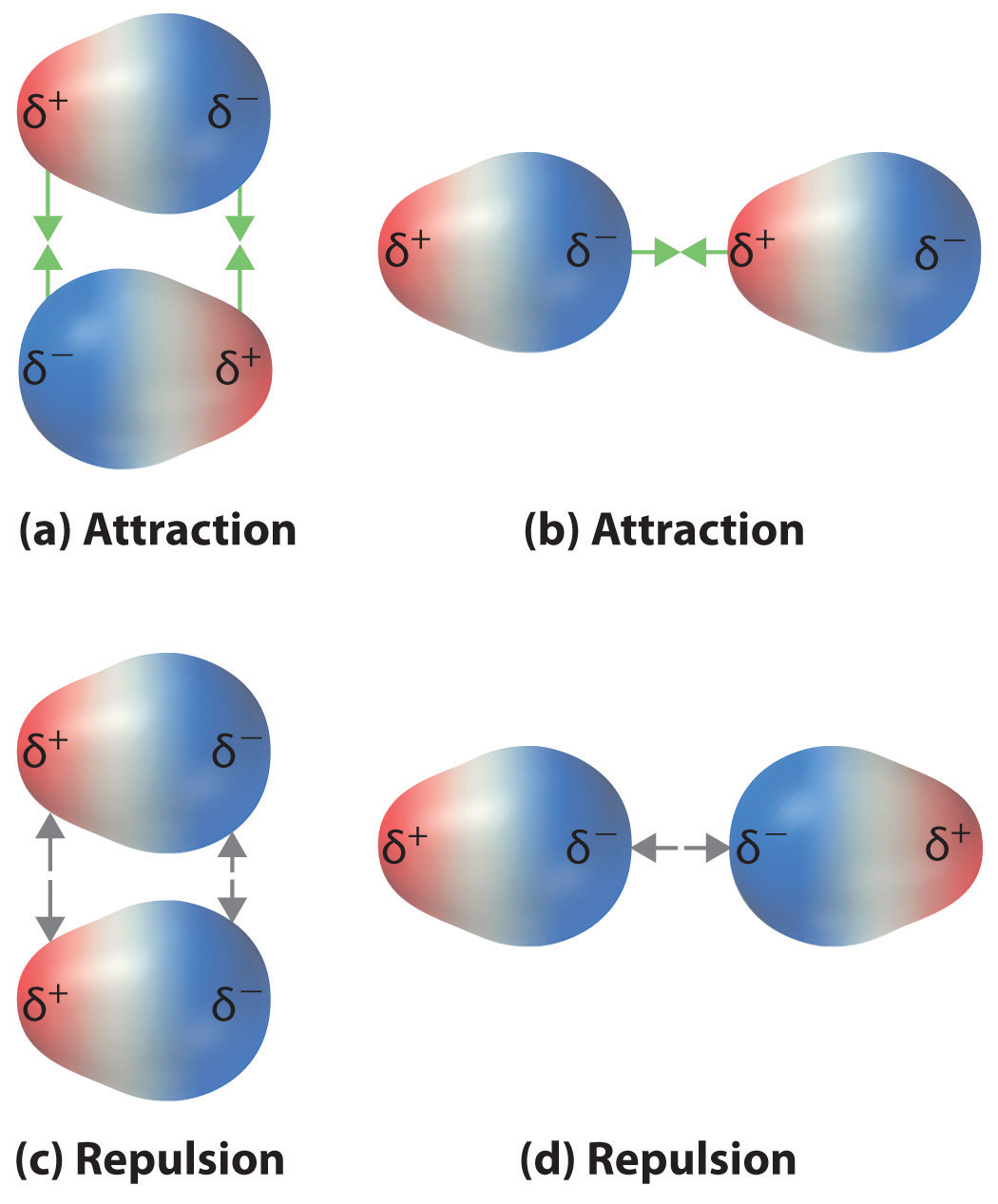
11.3 DipoleDipole Forces Chemistry LibreTexts
Dipole Moment — Definition & Overview Expii

How To Draw Overall Dipole Moment DRAWINGS OF LOVE
Web To Calculate The Dipole Moment Of A Chemical Bond, The Following Formula Is Used:
67 Views 6 Years Ago Chemistry Guided Notes.
Web The Dipole Moment Of A Molecule And Its Overall Polarity Depends On The Magnitude And Direction Of Individual Polar Bonds And Their Dipole Moments.
Web A Dipole Moment Is The Product Of The Magnitude Of The Charge And The Distance Between The Centers Of The Positive And Negative Charges.
Related Post: