Draw An Atom Of Oxygen
Draw An Atom Of Oxygen - Oxygen is in group 16/via, so it has six valence electrons. 6 valence electrons/atom × 1 atom = 6 + f: Hence, the oxygen atom has 6 valence electrons. The structures of h 2 , f 2 , and h 2 o would usually be drawn as follows: I started it off by having the students memorize the first 20 elements (h through ca), in their correct order — by atomic number — over their winter break. And, it can be shown in two ways: Web the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Memorization over the winter break. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. There is a double bond between oxygen atoms and two lone pairs exist on each oxygen atom. #1 first draw a rough sketch. Web valence electrons are the electrons in the outermost shell, or energy level, of an atom. Then place one dot at each side of the symbol. We’ll use a bohr diagram to visually represent where the electrons are around the. 4 valence electrons/atom} \times 1 \;\text{atom} & = 4 \\[1em] & \text{h: In the form of orbitals. However, this picture is at odds with the magnetic behavior of oxygen. A diagram of an oxygen atom. Web how do you draw an oxygen atom? 7 valence electrons/atom × 2 atoms = 14 ¯ = 20 valence electrons. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. Individual atoms are extremely small. State the modern atomic theory. In the form of orbitals. There are several interesting steps in drawing oxygen molecule's lewis structure. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. #2 mark lone pairs on the atoms. There is a double bond between oxygen atoms and two lone pairs exist on each oxygen atom. Energy p 4 enter orbital label continue. State the modern atomic theory. 126k views 12 years ago. The electron dot diagram for an element shows the valence electrons for the element. There is an o=o double bond, and each oxygen atom has eight electrons around it. 4 valence electrons/atom} \times 1 \;\text{atom} & = 4 \\[1em] & \text{h: The electronic configuration shows the distribution of electrons in an atom. There are now four unpaired electrons around the oxygen symbol. Hence, the oxygen atom has 6 valence electrons. Web the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Web valence electrons are the electrons in the outermost shell, or energy level, of an atom. For the purposes of this article, we'll use carbon as an example. Learn how atoms are constructed.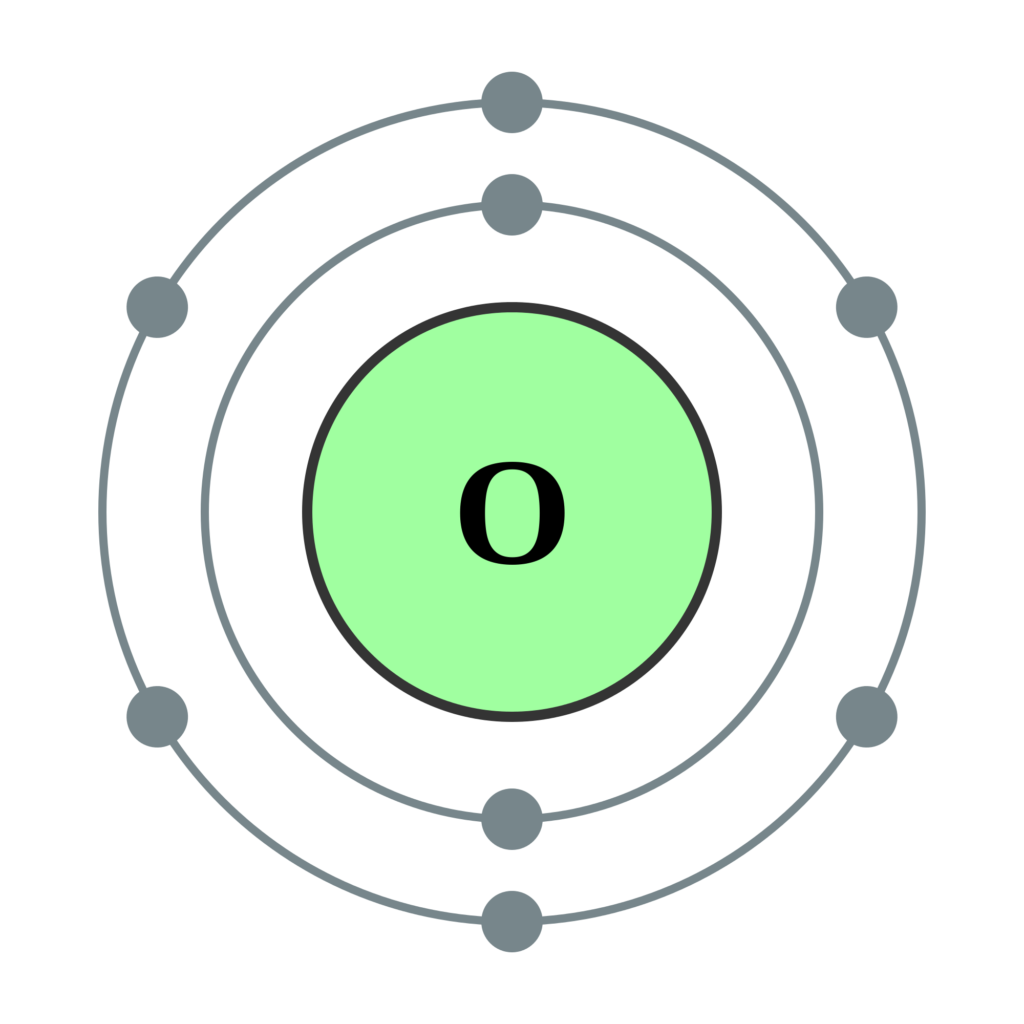
What is the Electron Configuration of Oxygen Archives Dynamic

Oxygen atom bohr model Royalty Free Vector Image
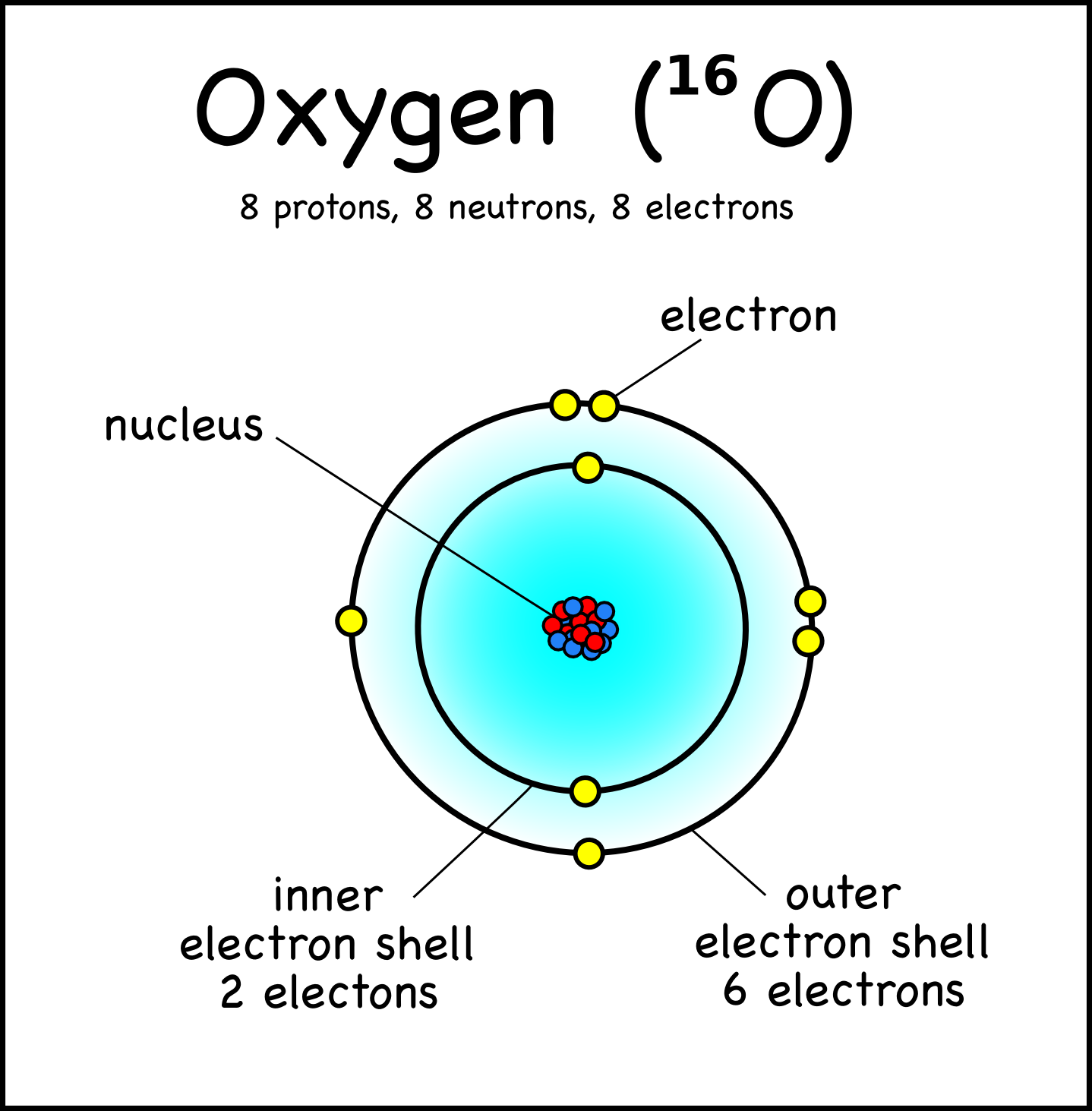
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Steps Of Drawing Lewis Structure Of O 2 Molecule.
This Problem Has Been Solved!
#5 Repeat Step 4 If Needed, Until All Charges Are Minimized, To Get A Stable.
Memorization Over The Winter Break.
Related Post: