Draw A Successive Ionization Energy Diagram For Aluminum
Draw A Successive Ionization Energy Diagram For Aluminum - By relative location, i mean correctly to. Web the successive ionization energy diagram is shown in the picture below. Web successive ionisation data can be used to: Web to draw a successive ionization energy diagram for aluminum, we will use the ionization energy data given on page 60. Web ionization energy chart of all the elements is given below. I 1 i_1 i 1 = 578 kj/mol. 3rd ionization energy, 2881 kj ⋅ mol−1. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the. Web for instance, the ionization energy of sodium (alkali metal) is 496kj/mol (1) whereas chlorine's first ionization energy is 1251.1 kj/mol (2). Web successive ionisation data can be used to: Predict or confirm the simple electronic configuration of elements. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13). Web values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element increase as they go; 3rd ionization energy, 2881. The first ionization energy is the. Web the successive ionization energy diagram is shown in the picture below. 3rd ionization energy, 2881 kj ⋅ mol−1. Web we can define a first ionization energy (i 1), a second ionization energy (i 2), and in general an nth ionization energy (i n) according to the following reactions: I 2 i_2 i 2. Confirm the number of electrons in the outer shell of an element. Web calculate the ph of a neutral aqueous solution at 0°c. Web values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element increase as they go; Ionisation energies and electron affinity. 4th ionization energy, 11600 kj ⋅. Web we can define a first ionization energy (i 1), a second ionization energy (i 2), and in general an nth ionization energy (i n) according to the following reactions: 4th ionization energy, 11600 kj ⋅. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the. Confirm the number of electrons in the outer shell. Web successive ionisation data can be used to: Describe and explain the observed trends in ionization energy of the elements. Web ionization energy chart of all the elements is given below. When you add 3p1, the outermost electron is in the 3p orbital (even though 3s and 3p are all valence electrons). On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13). Web calculate the ph of a neutral aqueous solution at 0°c. As could be expected from their electron. 2nd ionization energy, 1816 kj ⋅ mol−1; Electron affinity of aluminum is 42.5 kj/mol. Predict or confirm the simple electronic configuration of elements. I 1 i_1 i 1 = 578 kj/mol. From the picture, we can see that the fourth ionization energy has a much larger value than the.
Successive Ionisation Energy vigglegiggle
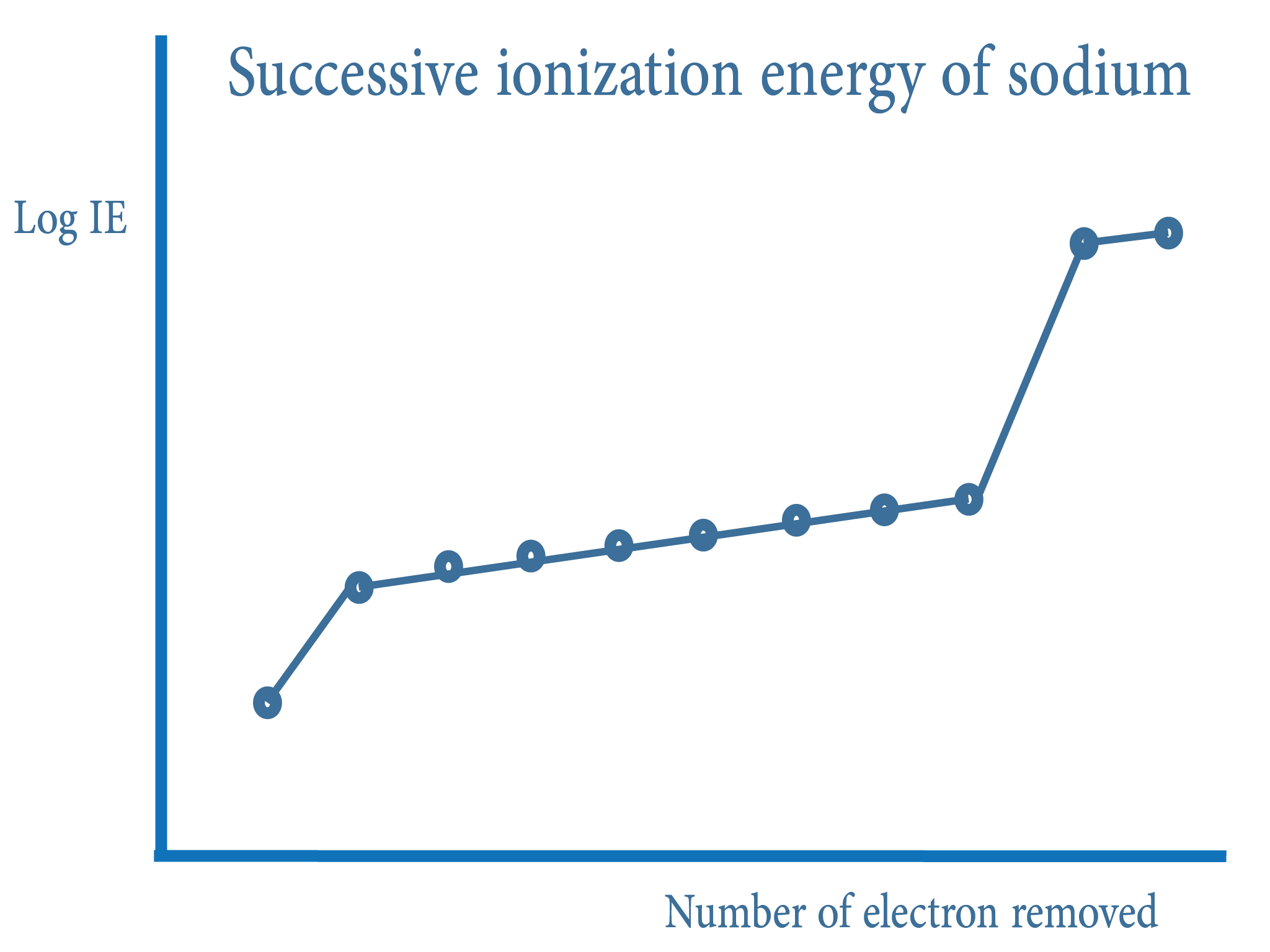
Ionisation Energy & Trends Revise Zone
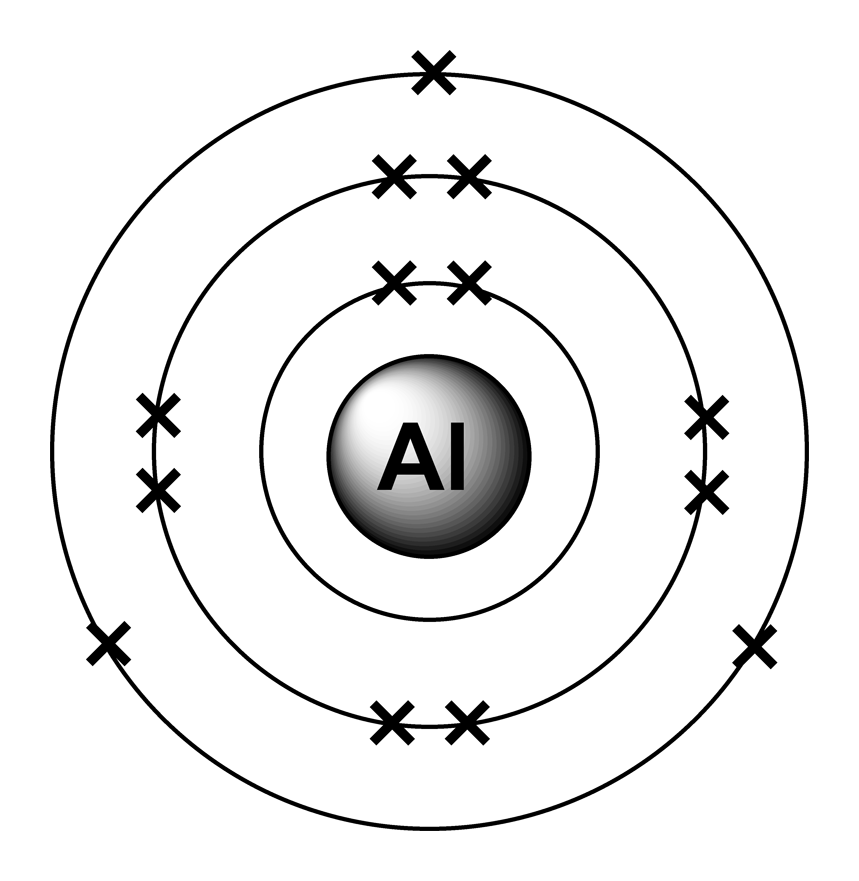
Electron arrangements
Web To Create A Successive Ionization Energy Diagram For Aluminum, We'll Focus On The First Few Ionization Energies:
Predict The Order Of Increasing Energy For The Following Processes:
First Ionization Energy, Second Ionization Energy As Well As Third Ionization Energy Of The Elements.
The Electron Affinity Of Aluminium Is 42.5 Kj Mol ‑1.
Related Post: