Draw A Sodium Atom
Draw A Sodium Atom - You can also play a fun. So, it's easy to see that the atom above contains two electrons. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) sodium (na) electron. Describe how electrons are grouped within atoms. This video shows how to draw the orbital diagram of sodium (na). Web draw the bohr diagram of an atom with 18 electrons or fewer. Web do you want to learn how to build an atom from scratch? This year, i’ve been basing my introduction to basic chemistry for my middle school students around the periodic table of the elements. The electronic configuration of sodium. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Web when referring to an atom, we simply use the element's name: Web draw the bohr diagram of an atom with 18 electrons or fewer. The term sodium refers to the element as well as an atom of sodium. Find the number of protons, electrons, and neutrons in the sodium atom. As we'll discuss later in the article, atomic. Although we have discussed the general arrangement of. Sodium electron configuration notation the configuration notation provides an easy way for. Web when referring to an atom, we simply use the element's name: Neutral sodium atom has 11 electrons whereas sodium ion ( n a +) has 10 electrons. Find the number of protons, electrons, and neutrons in the sodium atom. Protons are the positively charged. So, it's easy to see that the atom above contains two electrons. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. 32k views 9 years ago. Web when referring to an atom, we simply use the element's name: Web complete step by step answer: Sodium electron configuration notation the configuration notation provides an easy way for. Web when referring to an atom, we simply use the element's name: Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) sodium (na) electron. This video shows how to draw the orbital diagram of sodium (na). Although we have discussed the general arrangement of. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Sodium is an atom in the periodic table with atomic number. You can also play a fun. The electronic configuration of sodium. This year, i’ve been basing my introduction to basic chemistry for my middle school students around the periodic table of the elements. 140k views 5 years ago. The bohr model represents the particle nature of electrons. The electron shells are shown, moving outward from the nucleus. Neutral sodium atom has 11 electrons whereas sodium ion ( n a +) has 10 electrons. The term sodium refers to the element as well as an atom of sodium.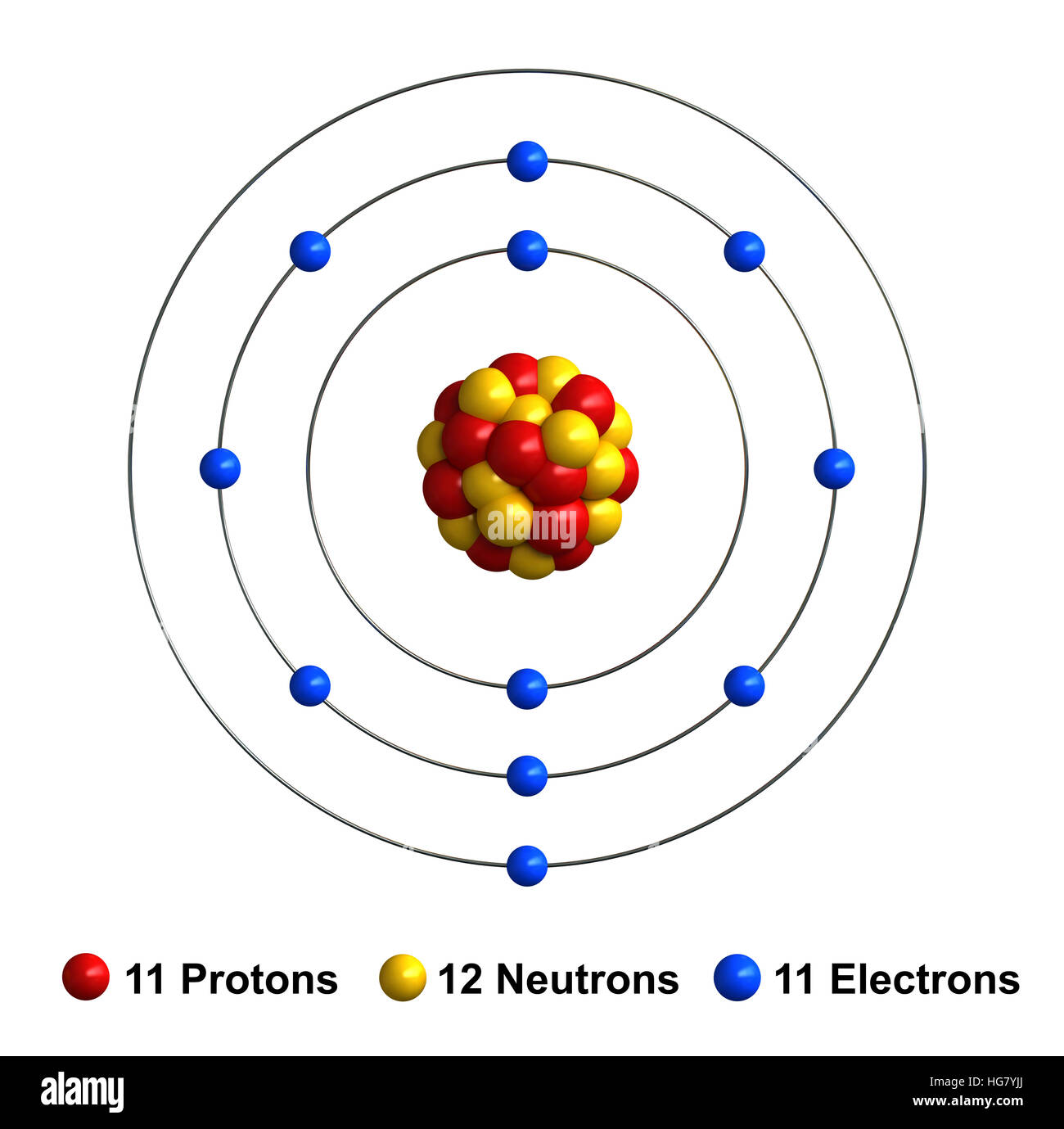
3d render of atom structure of sodium isolated over white background

Atomic Structure Of Sodium Ion

FileElectron shell 011 sodium.png Wikimedia Commons
Web Do You Want To Learn How To Build An Atom From Scratch?
Describe How Electrons Are Grouped Within Atoms.
Niels Bohr Proposed An Early Model Of The Atom As A Central Nucleus Containing Protons And.
As We'll Discuss Later In The Article, Atomic.
Related Post: