Draw A Lewis Structure For C2H4
Draw A Lewis Structure For C2H4 - Sum the valence electrons from all the atoms. Step method to draw the lewis dot structure of c 2 h 4 o. Write the correct skeletal structure for the molecule. Find the total valence electrons in c2h4 molecule. This molecule is also represented by h2c=ch2, clearly showing the alkene nature of the compound. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. #4 complete octet on central atom. Web the partial lewis structure that follows is for a hydrocarbon molecule. By the end of this section, you will be able to: Web this video teaches you how to draw the lewis structures and themoleculargeometry for ethylene (c2h4). Draw lewis structures for the following molecular formulas: For the c2h4 structure use the periodic table to find the total number of valence electrons for the. Web the molecular structure of c2h4 is often described as trigonal planar, as the carbon atoms and the attached hydrogen atoms are all in the same plane. Web this video teaches you how to. Ethene| c 2 h 4. Sum the valence electrons from all the atoms. In order to find the total valence electrons in c2h4 molecule, first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Let’s one by one discuss each step in detail. * hydrogen atoms are always terminal (only one bond). The chemical formula c2h4 represents ethylene. In order to find the total valence electrons in c2h4 molecule, first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Web lewis structure for c2h4. A review of general chemistry lewis structure. For the c2h4 structure use the periodic table to find the total number. Web how to draw the lewis dot structure for c2h4: For the c2h4 structure use the periodic table to find the total number of valence electrons for the. In reality, the molecular shape of ethene is not linear. Step method to draw the lewis dot structure of c 2 h 4 o. This planar geometry is a result of the electron density around the carbon atoms and. In the full lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the molecule. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. In order to find the total valence electrons in c2h4 molecule, first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. In order to draw the lewis structure of c2h4, first of all you have to find the total number of valence electrons present in the c2h4 molecule. This molecule is also represented by h2c=ch2, clearly showing the alkene nature of the compound. C2h4 lewis structure, molecular structure, hybridization, bond angle and shape. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Find the total valence electrons in c2h4 molecule. Web use these steps to correctly draw the c 2 h 4 lewis structure: Note that the c 2 h 4 lewis dot structure involves sharing more than one pair of electrons.
C2h4 Dot Diagram
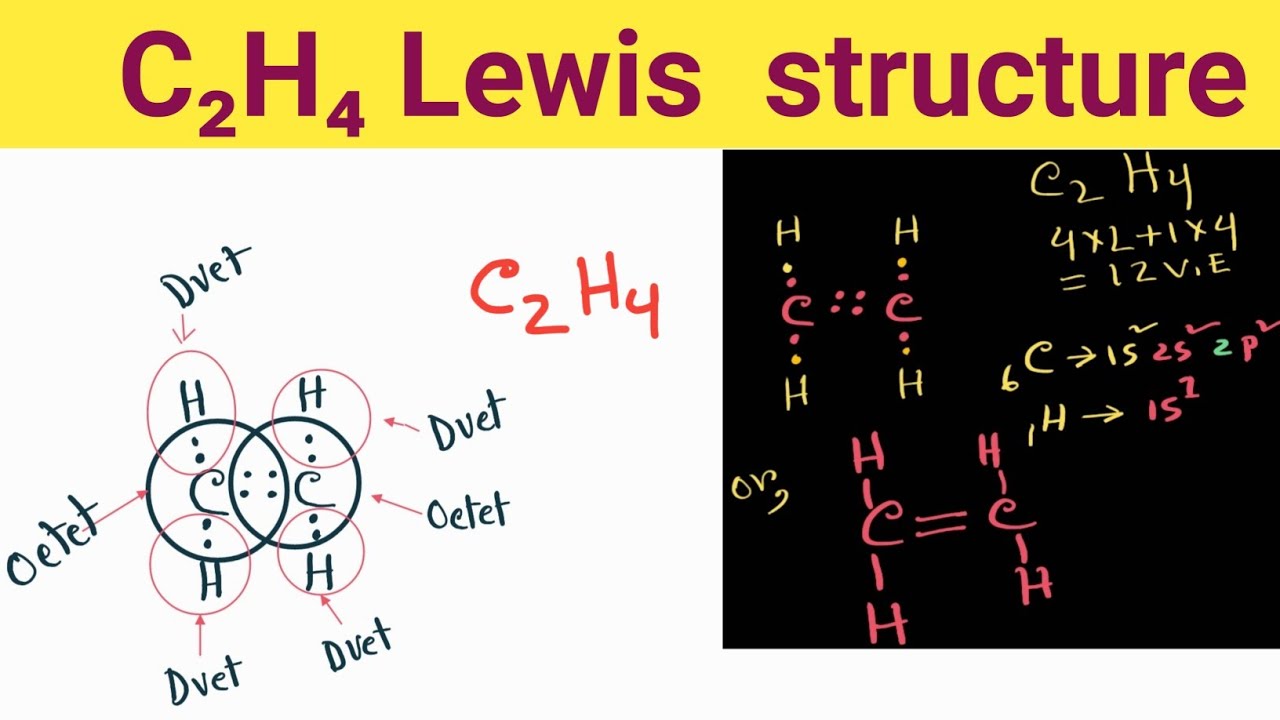
C2H4 Lewis StructureHow do you draw the Lewis structure for C2H4

C2H4 Lewis Structure (Ethylene) YouTube
Let’s One By One Discuss Each Step In Detail.
Hybridization Of Atoms In Ethene Molecue Can Be Found From Lewis Structure.
Web The Molecular Structure Of C2H4 Is Often Described As Trigonal Planar, As The Carbon Atoms And The Attached Hydrogen Atoms Are All In The Same Plane.
Web Lewis Structure For C2H4.
Related Post: