Dehydration Synthesis Drawing
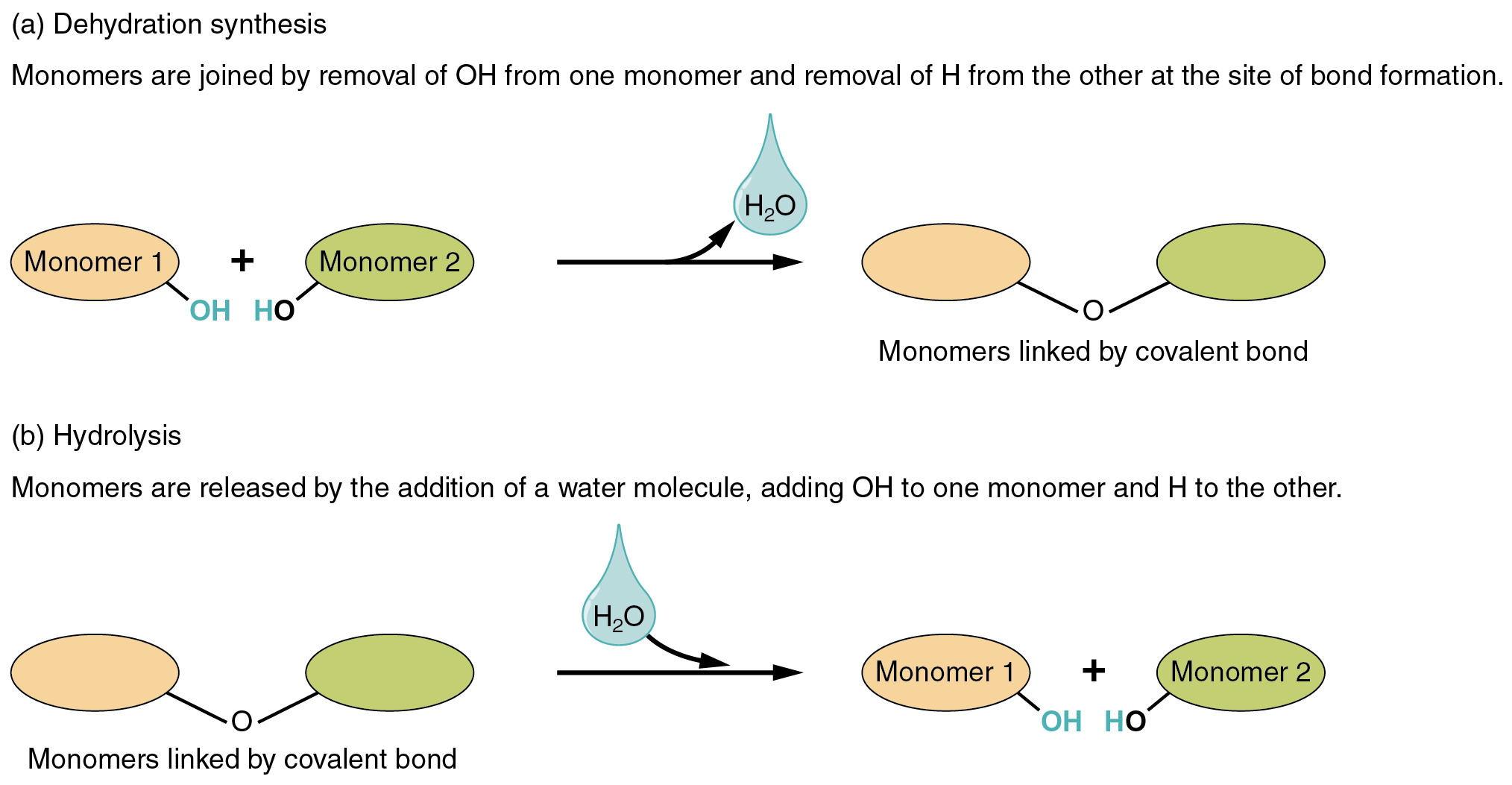
Dehydration Synthesis Drawing - Web each bond forms in a dehydration synthesis (condensation) reaction. In hydrolysis reactions, a water molecule is consumed as a result of breaking the covalent bond holding together two components of a polymer. Dehydration reactions are common processes, the reverse of a hydration reaction. Web drawing macromolecule interactions (10 pts) define dehydration synthesis aka condensation reaction p. Web in the dehydration synthesis reaction between two molecules of glucose, a hydroxyl group from the first glucose is combined with a hydrogen from the second glucose, creating a covalent bond that links the two monomeric sugars (monosaccharides) together to form the dissacharide maltose. Dehydration reactions in organic chemistry. Web dehydration synthesis or a condensation reaction (video) | khan academy. The monosaccharide glucose can be used as a building block for more complex sugars and carbohydrates. In the dehydration synthesis reaction above, two molecules of the sugar glucose (monomers) combine to form a single molecule of the sugar maltose. Web the diagram below illustrates how dehydration synthesis generally occur in polymers. Web key areas covered. Web in chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. What is the difference between dehydration synthesis and hydrolysis. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Structure and synthesis of alkenes. In this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another, releasing a molecule of water and forming a covalent bond known as a glycosidic. Most macromolecules are made from single subunits, or building blocks, called monomers. The resulting bond between amino acids is a peptide bond Web normally, when we refer to this process in. Web key areas covered. Web biology library > macromolecules > proteins. Two glucose molecules can be linked together through a dehydration synthesis reaction to form a disaccharide called maltose. Alkene synthesis by dehydration of alcohols. The resulting bond between amino acids is a peptide bond In your organic chemistry or biology class, you will come across the terms dehydration synthesis and hydrolysis. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis refers to the formation of larger molecules from smaller reactants, accompanied by the loss of a water molecule. Find more free tutorials, videos and readings for the science classroom at ricochetscience.com. Web in chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Most macromolecules are made from single subunits, or building blocks, called monomers. Web normally, when we refer to this process in biology, we call it a «dehydration synthesis», since we are building a higher order structure at the expense of a loss of water. 37) draw and label hydrolysis of a polymer carbohydrate (10 pts) p. Web each bond forms in a dehydration synthesis (condensation) reaction. Web biology library > macromolecules > proteins. In the dehydration synthesis reaction above, two molecules of the sugar glucose (monomers) combine to form a single molecule of the sugar maltose. In this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another, releasing a molecule of water and forming a covalent bond known as a glycosidic. The resulting bond between amino acids is a peptide bond Alkene synthesis by dehydration of alcohols. During protein synthesis, the carboxyl group of the amino acid at the end of the growing polypeptide chain chain reacts with the amino group of an incoming amino acid, releasing a molecule of water. 765k views 9 years ago.
Dehydration Synthesis
Compounds Essential to Human Functioning · Anatomy and Physiology
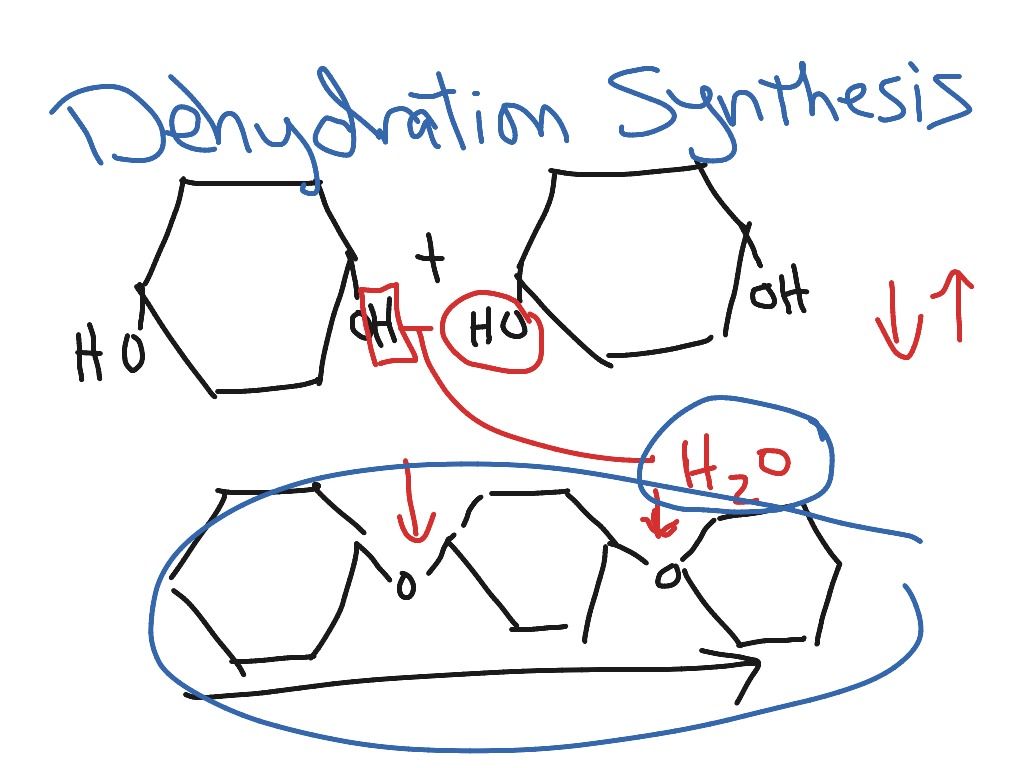
Dehydration synthesis glucose Chemistry ShowMe
In Hydrolysis Reactions, A Water Molecule Is Consumed As A Result Of Breaking The Covalent Bond Holding Together Two Components Of A Polymer.
Web Dehydration Synthesis Involves The Formation Of New Chemical Bonds Between Two Molecules Which Leads To The Formation Of New Compounds.
Want To Join The Conversation?
Use Your Models Of Glucose Molecules To Show What Happens When They Undergo Dehydration Synthesis.
Related Post: