Atomic Number Drawing
Atomic Number Drawing - So there are 92 electrons and then finally, to figure out the number of neutrons, we subtract this number from the mass number. The circle with the c is a representative nucleus, so now you'll need to indicate the electron orbitals. It is denoted by the symbol z and is the subscript in atomic notation. Web in this lesson i present an overview of: Since they memorized the elements in order, they should be able to figure this out on their own — but they could also look it up quickly on the periodic table, or look at the element symbol where the atomic number is sometimes written on the lower left. State the modern atomic theory. Web steps for constructing an orbital diagram. As an example, we will use argon, whose atomic number is 18 and electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6. Suitable for all abilities including sen and a logical method for pupils with asd. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The power point also has tasks in it for students to complete. The atomic number is also called the proton number. Web define atomic and mass numbers. The development of modern atomic theory revealed much about the inner structure of atoms. Web the information needed to draw atomic diagrams is found on the periodic table. Web so once again for protons, we look at the atomic number, that's 92. Learn how atoms are constructed. So there are 92 electrons and then finally, to figure out the number of neutrons, we subtract this number from the mass number. Web updated on november 05, 2019. The smallest piece of an element that maintains the identity of that. Web in this lesson i present an overview of: Suitable for all abilities including sen and a logical method for pupils with asd. Which is also the number of electrons in a neutral atom. The symbol z comes from the german word zahl, which means numeral, or atomzahl, which means. So there are 92 electrons and then finally, to figure. Hydrogen has 1 proton and no neutrons. So there must be 92 protons. Web in this lesson i present an overview of: Learn how atoms are constructed. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). Atomic diagrams (using the periodic table) n atomic number (z) = number of protons. A primer on what an atom is from fuse school chemistry. A primer on subatomic particles and atoms. Label the location of subatomic particles in the atom. The symbol z comes from the german word zahl, which means numeral, or atomzahl, which means. Web the information needed to draw atomic diagrams is found on the periodic table. Counting protons, electrons, and neutrons get 5 of 7 questions to level up! For example, potassium has 19 electrons draw a small circle and write the symbol in the centre. Web simple rules to guide you to draw atomic structures! Web protons = 6: So there are 92 electrons and then finally, to figure out the number of neutrons, we subtract this number from the mass number.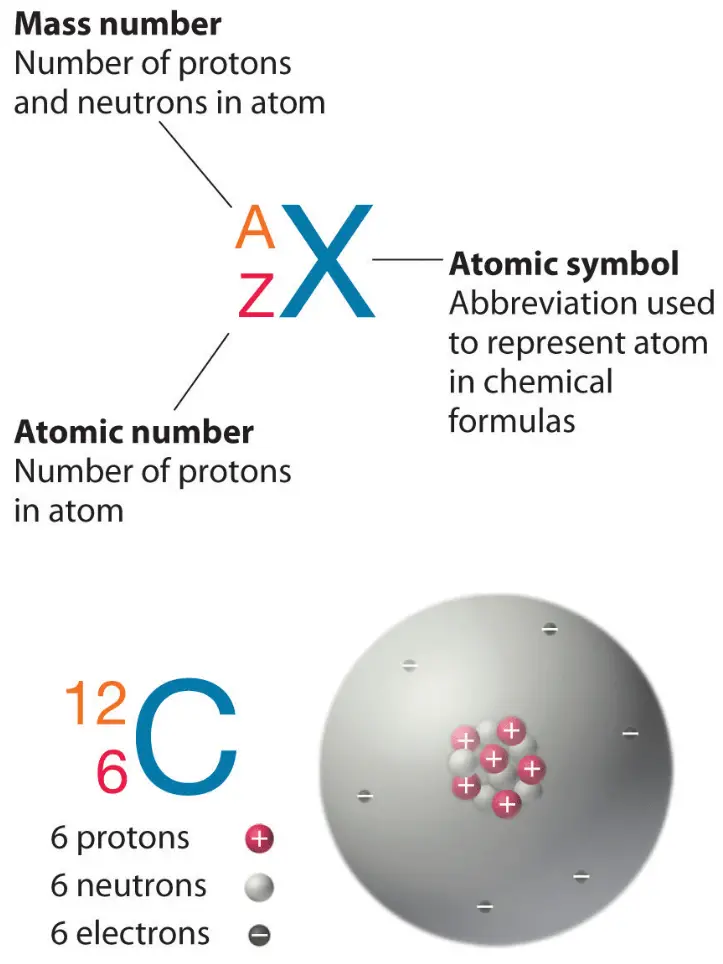
Atomic Mass Number Definition & Characteristics

List of Elements By Atomic Number
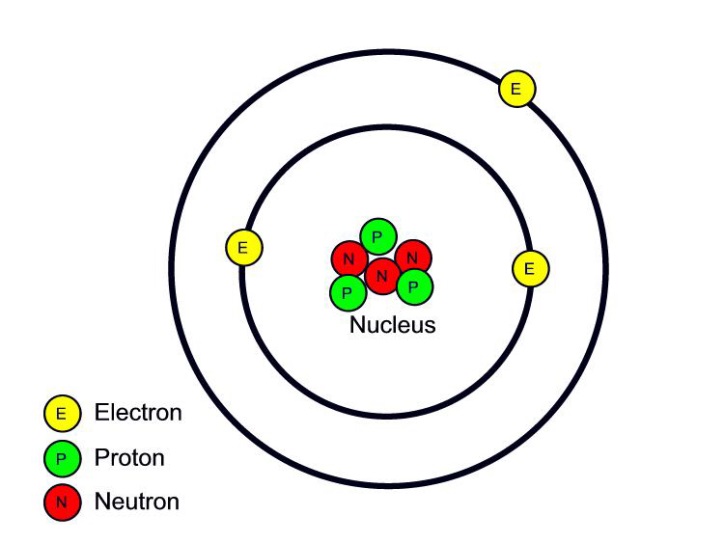
Atomic structure WGHS Junior Science
Suitable For All Abilities Including Sen And A Logical Method For Pupils With Asd.
Visualize Trends, 3D Orbitals, Isotopes, And Mix Compounds.
The Development Of Modern Atomic Theory Revealed Much About The Inner Structure Of Atoms.
Since We Know The Atomic Number Is 6 (Because We Memorized It), The Atom Has 6 Protons.
Related Post: